Recombinant pichia pastoris, construction method and application of recombinant pichia pastoris
A Pichia pastoris, gs115-ppic9-novq technology, applied in the field of recombinant Pichia pastoris, can solve the problems of low expression amount, complicated purification process, etc., and achieves high catalytic activity, low substrate specificity, and simplification. The effect of the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A recombinant strain of Pichia pastoris (GS115-ppic9-novQ), which was preserved in the China Center for Type Culture Collection (CCTCC) on March 11, 2016, address: 299 Bayi Road, Luojia Mountain, Wuchang District, Wuhan City, Hubei Province No., China Center for Type Culture Collection), the deposit number is CCTCC M 2016105.
[0035] Constructed by the following steps:
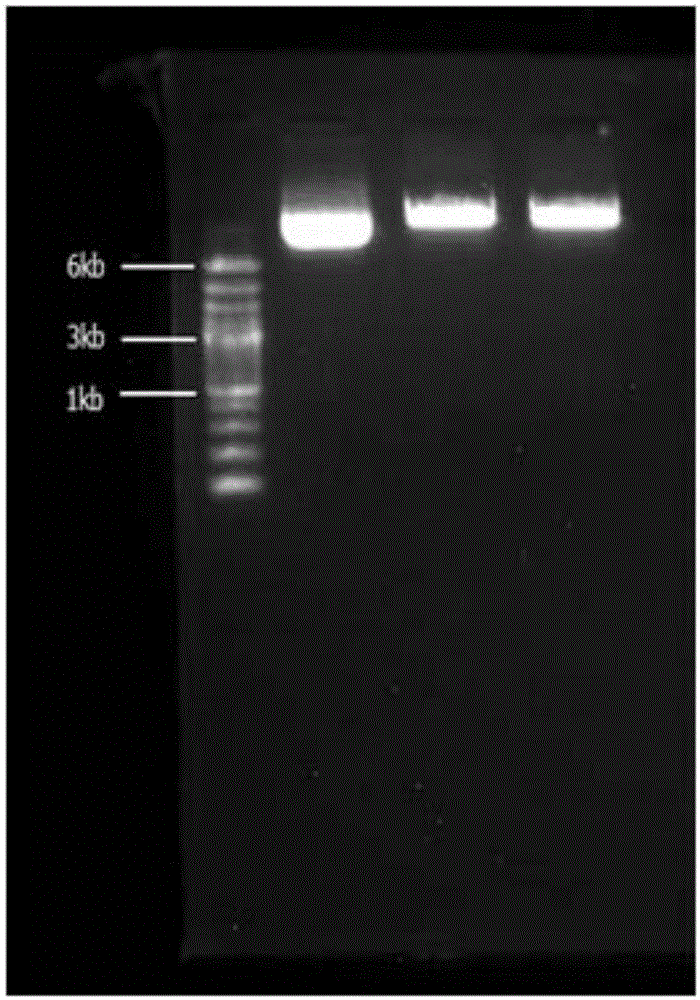
[0036] Step 1: Construction of recombinant Pichia pastoris expression vector ppic9-novQ:
[0037] (1) Prenyl transferase novQ gene acquisition
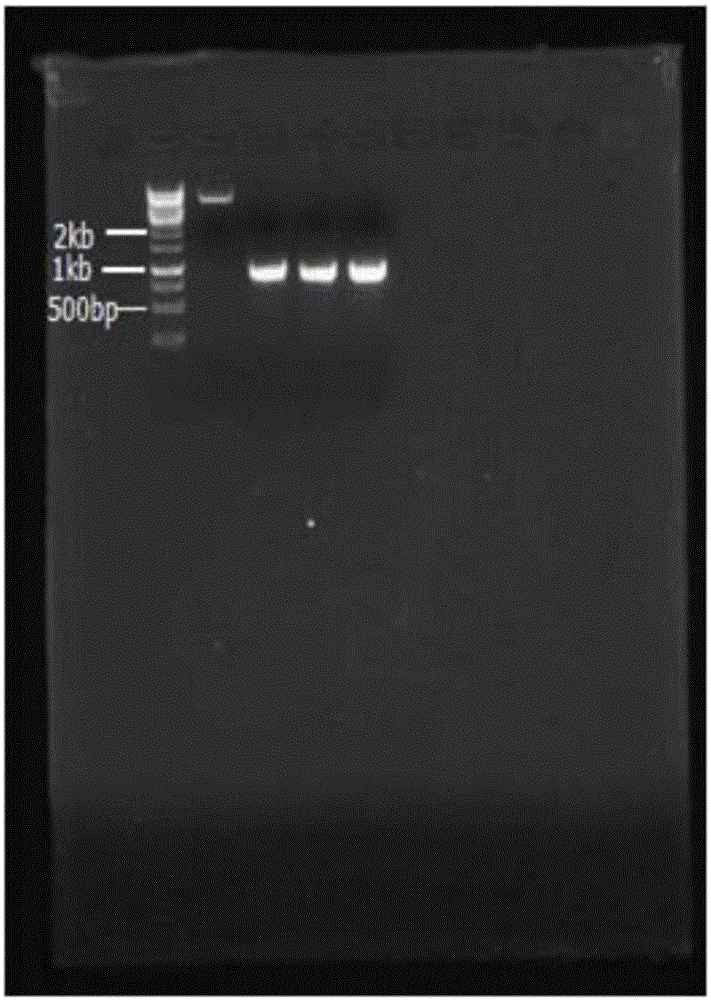
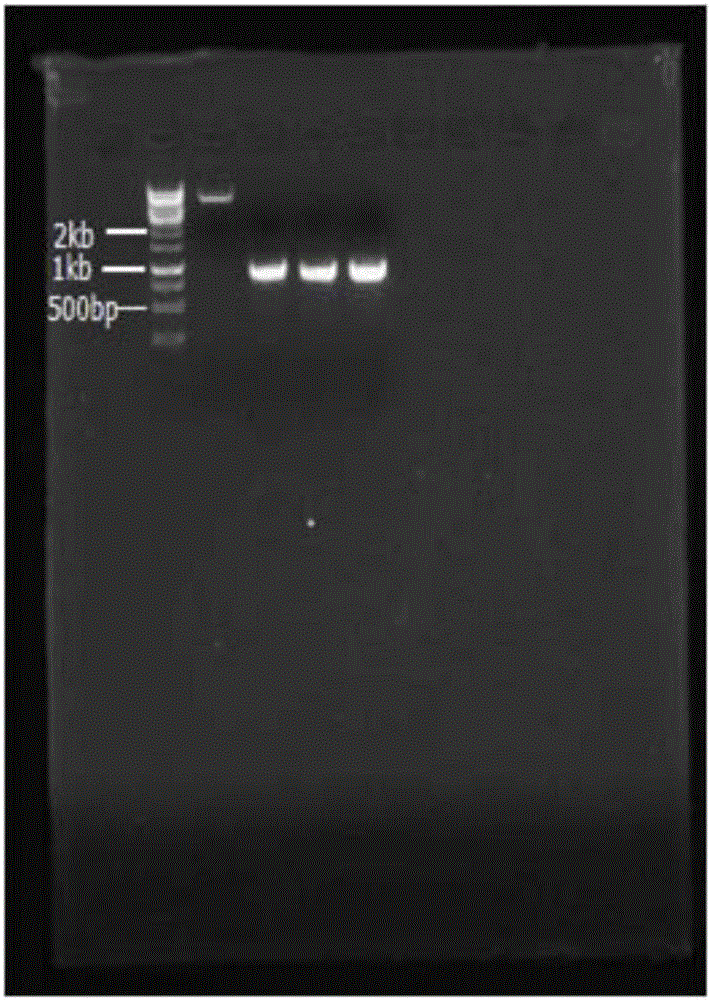
[0038] Use Gao's No. 1 medium, 50mL / 250mL liquid volume, 28°C, 180rpm shake flask to culture Streptomyces snow white (purchased from China Pharmaceutical Microorganism Culture Collection Management Center) for 72h, filter to obtain mycelia, and use the CTAB method to extract bacteria Total silk DNA. Using the total DNA as a template, PCR amplification was performed using the NovQ gene forward primer SEQ ID No.1 and reverse primer SEQ ID No.2. The amplificatio...
Embodiment 2
[0049] A recombinant Pichia pastoris GS115-ppic9-novQ is used to produce aromatic prenyl transferase NovQ, comprising the following steps:
[0050] (1) Pick a single colony of recombinant Pichia pastoris GS115-ppic9-novQ, inoculate it in a 250mL Erlenmeyer flask filled with 25mL of MGY medium, and cultivate it at 28°C with shaking at 250rpm for 36h to obtain activated recombinant Pichia pastoris GS115 - ppic9-novQ bacteria solution.
[0051] (2) The activated recombinant Pichia pastoris GS115-ppic9-novQ bacterial solution was inserted into a 250 mL Erlenmeyer flask filled with 25 mL of BMGY medium at an inoculum volume of 10% by volume, and cultured at 28°C and 250 rpm for 36 hours with shaking. The fermentation broth was centrifuged at 4000 g for 15 min to remove the culture medium to obtain recombinant Pichia pastoris GS115-ppic9-novQ cells.
[0052] (3) Resuspend the recombinant Pichia pastoris GS115-ppic9-novQ bacteria in the same volume of BMMY medium as before centrifug...
Embodiment 3
[0055] A recombinant Pichia pastoris GS115-ppic9-novQ is used to produce aromatic prenyl transferase NovQ, comprising the following steps:
[0056] (1) Pick a single colony of recombinant Pichia pastoris GS115-ppic9-novQ, inoculate it in a 250mL Erlenmeyer flask filled with 12.5mL of BMGY medium, culture at 20°C, 180rpm shaking for 24 hours, and obtain activated recombinant Pichia pastoris GS115-ppic9-novQ bacterial fluid.
[0057] (2) The activated recombinant Pichia pastoris GS115-ppic9-novQ bacterial solution was inserted into a 250mL Erlenmeyer flask containing 12.5mL MGY medium at an inoculum volume of 5% by volume, and cultured at 20°C and 180rpm for 24h with shaking. The fermentation broth was centrifuged at 2000 g for 10 min to remove the culture medium to obtain recombinant Pichia pastoris GS115-ppic9-novQ cells.
[0058] (3) Resuspend the recombinant Pichia pastoris GS115-ppic9-novQ in the same volume of BMMY medium as before centrifugation, 12.5mL / 250mL liquid volu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com