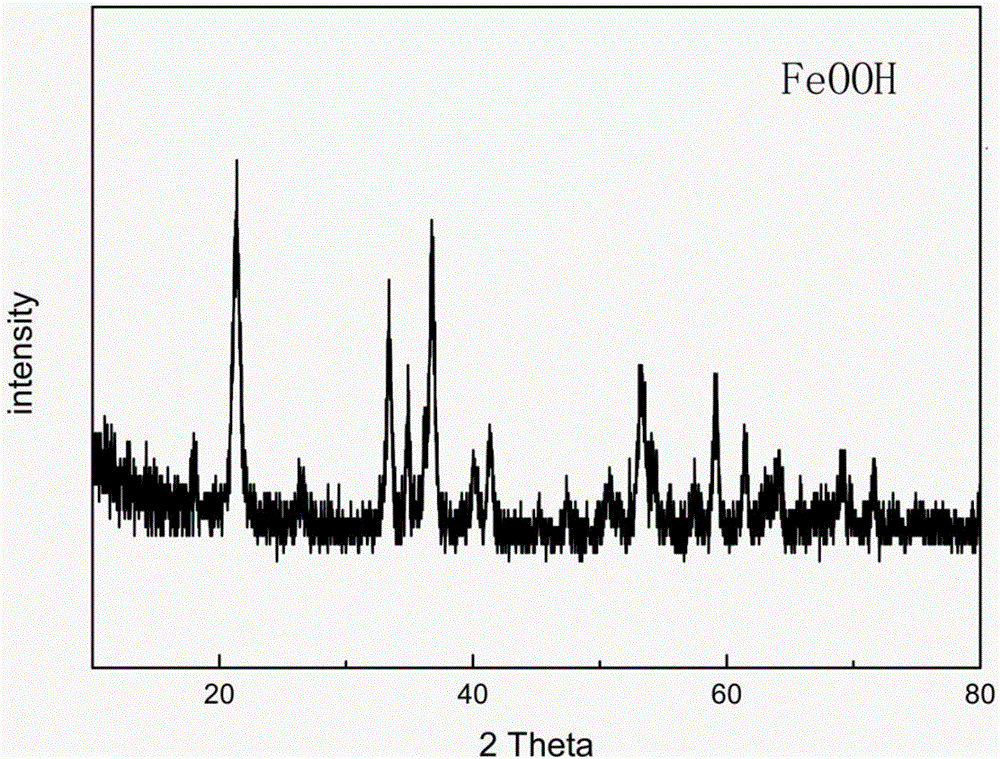
Method for preparing iron oxyhydroxide nanosheet and its product
A technology of iron oxyhydroxide and nanosheets, which is applied in the direction of iron oxide/hydroxide, etc., can solve the problems of large size of nanosheets, failure to inhibit the growth of FeOOH crystals, and difficult removal of impurity ions, etc., to achieve large specific surface area, easy to control, growth inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Weigh 10mmol of ferric nitrate nonahydrate, dissolve it in 35ml of deionized water, and stir to an orange clear solution;
[0029] 2) Weigh 20 mmol of tetramethylammonium hydroxide, add it into the solution prepared in step 1), and stir well to obtain a reddish-brown suspension.
[0030] 3) Add the suspension prepared in step 2) into the liner of the reaction kettle. Use deionized water to adjust its volume to account for 4 / 5 of the inner tank volume of the reactor to obtain a suspension of the reaction precursor. At this time, the molar ratio of Fe / TMAH is 1:2, Fe 3+ The molar concentration is 0.25mol / L.
[0031] 4) Seal the liner of the reaction kettle containing the reactant body in the reaction kettle, and heat it at 100° C. for 6 hours to carry out hydrothermal treatment. Then the reaction kettle was placed in the air and naturally cooled to room temperature, the reactant was taken out, filtered, washed with deionized water and ethanol in sequence, and dried ...
Embodiment 2
[0035] 1) Weigh 10mmol of ferric nitrate nonahydrate, dissolve it in 35ml of deionized water, and stir to an orange clear solution;
[0036] 2) Weigh 40mmol tetramethylammonium hydroxide, add it into the solution prepared in step 1), and stir fully to obtain a reddish-brown suspension;
[0037] 3) Add the suspension prepared in step 2) into the liner of the reaction kettle. Use deionized water to adjust its volume to account for 4 / 5 of the inner tank volume of the reactor to obtain a suspension of the reaction precursor. At this time, the molar ratio of Fe / TMAH is 1:4, Fe 3+ The molar concentration is 0.25mol / L;
[0038] 4) Seal the liner of the reaction kettle containing the reactant body in the reaction kettle, and heat it at 110° C. for 6 hours to carry out hydrothermal treatment. Then the reaction kettle was placed in the air and naturally cooled to room temperature, the reactant was taken out, filtered, washed with deionized water and ethanol in sequence, and dried at ...
Embodiment 3
[0040] 1) Weigh 10mmol of ferric nitrate nonahydrate, dissolve it in 35ml of deionized water, and stir to an orange clear solution;
[0041] 2) Weigh 60 mmol of tetramethylammonium hydroxide, add it to the solution prepared in step 1), and stir fully to obtain a reddish-brown suspension;
[0042] 3) Add the suspension prepared in step 2) into the liner of the reaction kettle. Use deionized water to adjust its volume to account for 4 / 5 of the inner tank volume of the reactor to obtain a suspension of the reaction precursor. At this time, the molar ratio of Fe / TMAH is 1:6, Fe 3+ The molar concentration is 0.25mol / L.
[0043]4) Seal the liner of the reaction kettle containing the reactant body in the reaction kettle, and heat it at 120° C. for 6 hours to carry out hydrothermal treatment. Then the reaction kettle was placed in the air to cool down to room temperature naturally, the reactant was taken out, filtered, washed with deionized water and ethanol in turn, and dried at 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com