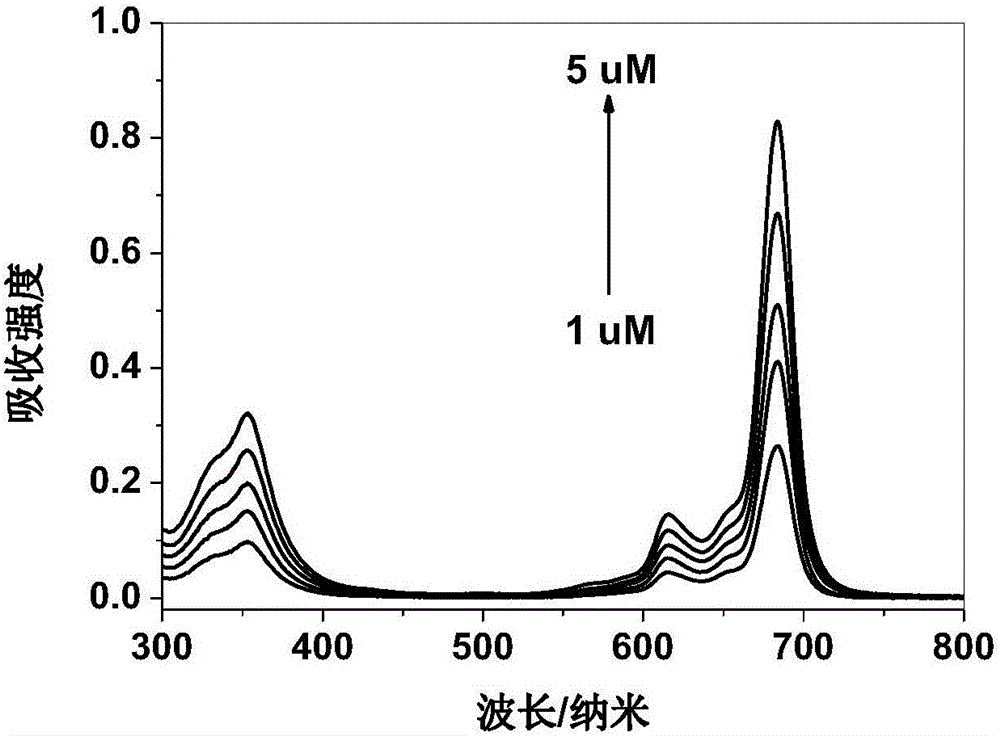
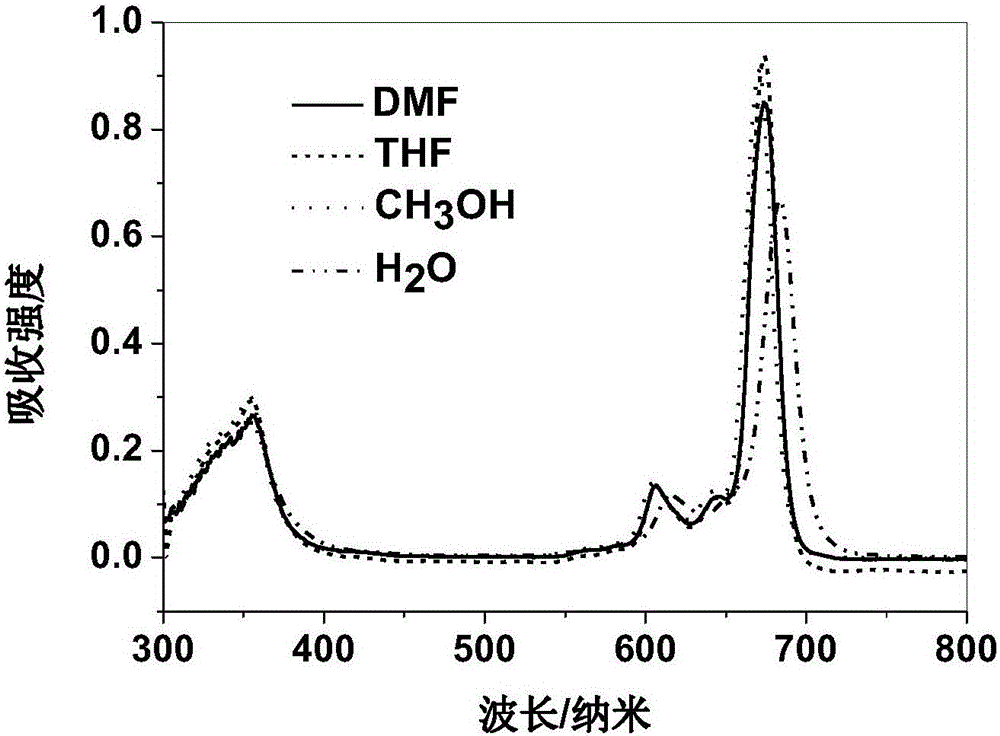
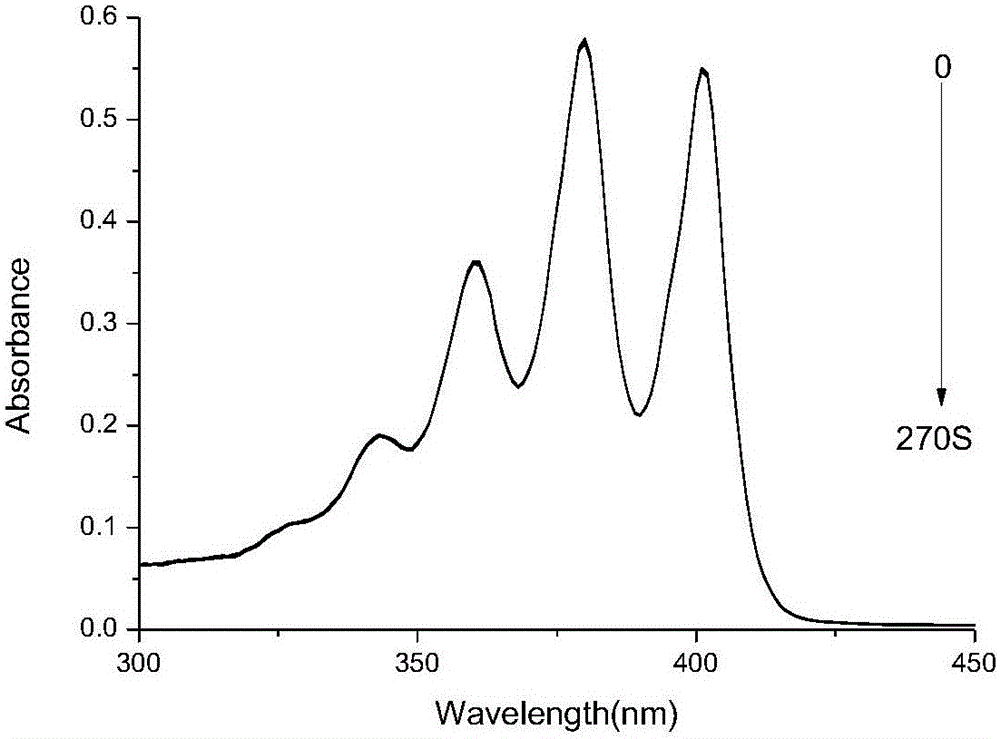
Axially substituted silicon phthalocyanine, method for synthesizing same and application of axially substituted silicon phthalocyanine to photodynamic therapy
A silicon phthalocyanine, axial technology, applied in the field of photosensitizers, can solve the problems of easy aggregation, affecting the photosensitization of phthalocyanines, reducing singlet oxygen quantum yield and fluorescence lifetime, etc. Aggregation, the effect of a simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Under nitrogen protection, in a 50mL three-necked flask equipped with a reflux device, phthalocyanine silicon dichloride (purchased from Sigma-Aldrich, USA, purity 85%) 50mg, 4-piperidinemethanol 37.67mg and catalyst NaH 30mg were added to In 15mL of toluene, heated to 110°C and refluxed for 24h. After the reaction, the organic solvent was evaporated under reduced pressure, and the product was dissolved in dichloromethane and washed to obtain 30.2 mg of 4-piperidinemethoxy-substituted silicon phthalocyanine, with a yield of 68%.
[0032] 1 H NMR (400MHz, CDCl 3 ,ppm): δ9.65(m,8H,Pc-Ha),8.35(m,8H,Pc-Hb),1.2-1.88(m,8H,CH 2 ),1.19(t,2H,NH).-1.10-1.56(m,8H,CH 2 ),-1.8,(m,2H,CH),-2.3(d,4H.CH 2 );
[0033] 13 C NMR: δ149.2, 135.9, 130.7, 123.66, 60.6, 45.0, 35.2, 27.8; IR: 3439, 2964, 2896, 1630, 1548, 1438, 1418, 1369, 1342, 1246, 1074, 1012, 909, 799cm -1 .
Embodiment 2
[0035]Under nitrogen protection, in a 50 mL three-necked flask equipped with a reflux device, 50 mg of silicon phthalocyanine dichloride, 47.09 mg of 4-piperidine methanol and 30 mg of catalyst NaH were added to 15 mL of toluene, and heated to 110 °C for reflux reaction for 24 h. After the reaction, the organic solvent was evaporated under reduced pressure, and the product was dissolved in dichloromethane and washed to obtain 26.8 mg of the product, 4-piperidinemethoxy-substituted silicon phthalocyanine, with a yield of 60.6%.
Embodiment 3
[0037] Under nitrogen protection, in a 50 mL three-necked flask equipped with a reflux device, 50 mg of silicon phthalocyanine dichloride, 75.34 mg of 4-piperidine methanol and 40 mg of catalyst NaH were added to 15 mL of toluene, and heated to 110 °C for reflux reaction for 24 h. After the reaction, the organic solvent was evaporated under reduced pressure, and the product was dissolved in dichloromethane and washed to obtain 21,2 mg of the product, 4-piperidinemethoxy-substituted silicon phthalocyanine, with a yield of 47.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com