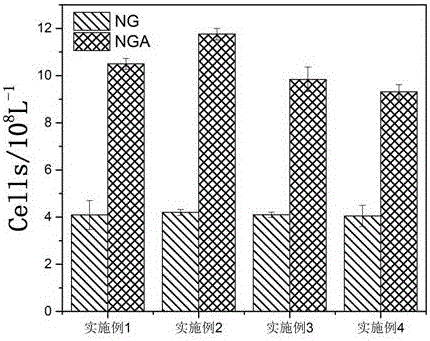
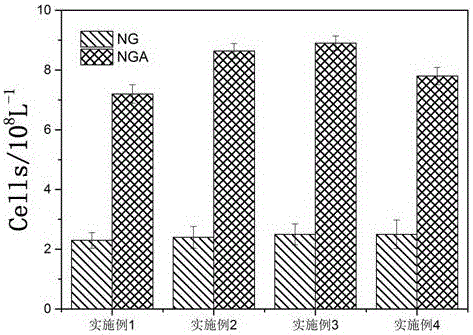
Acetylated naringin composition and medical application of composition in osteoblast proliferation
A technology for acetylating pomelo and a compound, which is applied in the fields of enzyme synthesis and medical application, can solve the problems of naringin not easily permeating the lipid cell membrane of the bilayer, reducing the effect of drugs, poor fat solubility, etc., so as to promote osteogenesis. Cell proliferation, simple synthesis process, and the effect of promoting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
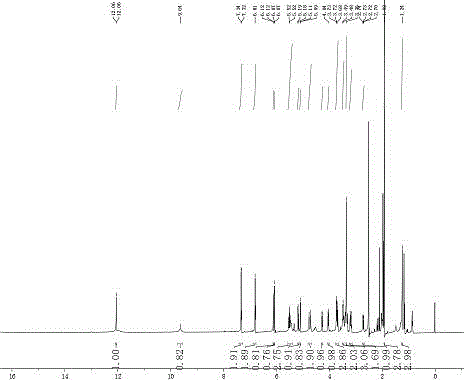
[0025] Weigh 0.05mmol of naringin and dissolve it in 20mL of acetone solution, add 2mmol of vinyl acetate, mix well, then add 80mg of CSL enzyme, place in a constant temperature shaker at 30°C, 100rpm, keep the water activity at 0.60, and react for 24h . At the end of the reaction, the enzyme was filtered to terminate the reaction and the solvent was concentrated by spin. The reaction product was separated and purified by HPLC. The separation conditions were: Thermo C18 (4.6 mm × 250 mm × 5 μm, Agilent, USA) chromatographic column; the mobile phase was water (A) and methanol (B), 0-5min 40-80% ( B), 5-15min maintain 80% (B), 15-20min 80-40% (B); flow rate 1.0 ml / min. The column temperature was 30°C, the injection volume was 10 µl; the detection wavelength was 330 nm, the retention time of acetylated naringin was 10.8 min, and the yield was 89.0%. The structure of the product was identified by nuclear magnetic resonance spectrometer, and it was determined that the substance s...
Embodiment 2
[0027] Weigh 0.2mmol of naringin and dissolve it in 20mL of acetone solution, add 4mmol of vinyl acetate, mix well, then add 100mg of CSL enzyme, place in a constant temperature shaker at 30°C, 100rpm, keep the water activity at 0.60, and react for 24h . At the end of the reaction, the enzyme was filtered to terminate the reaction and the solvent was concentrated by spin. The reaction product was separated and purified by HPLC. The separation conditions were: Thermo C18 (4.6 mm × 250 mm × 5 μm, Agilent, USA) chromatographic column; the mobile phase was water (A) and methanol (B), 0-5min 40-80% ( B), 5-15min maintain 80% (B), 15-20min 80-40% (B); flow rate 1.0 ml / min. The column temperature was 30°C, the injection volume was 10 µl; the detection wavelength was 330 nm, the retention time of acetylated naringin was 10.8 min, and the yield was 92.6%. The structure of the product was identified by nuclear magnetic resonance spectrometer, and it was determined that the substance s...
Embodiment 3
[0029]Weigh 0.4mmol of naringin and dissolve it in 20mL of acetonitrile solution, add 6mmol of vinyl acetate, mix well, add another 120mg of CSL enzyme, place in 30°C, 100rpm constant temperature shaker, keep water activity 0.60, react for 24h . At the end of the reaction, the enzyme was filtered to terminate the reaction and the solvent was concentrated by spin. The reaction product was separated and purified by HPLC. The separation conditions were: Thermo C18 (4.6 mm×250 mm×5 μm, Agilent, USA) chromatographic column; the mobile phase was water (A) and methanol (B), 0-5min 40-80% ( B), 5-15min maintain 80% (B), 15-20min 80-40% (B); flow rate 1.0 ml / min. The column temperature was 30°C, the injection volume was 10 µl; the detection wavelength was 330 nm, the retention time of acetylated naringin was 10.8 min, and the yield was 92.0%. The structure of the product was identified by nuclear magnetic resonance spectrometer, and it was determined that the substance synthesized by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com