Preparation method of low-cost high-performance lithium iron phosphate material
A lithium iron phosphate, high-performance technology, applied in the field of preparation of lithium iron phosphate materials, can solve the problems affecting the cycle capacity and battery rate charge and discharge performance, affect the conductivity, high price and cost, and achieve the improvement of cycle capacity and rate charge and discharge Performance, simple preparation method, and the effect of reducing the total cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
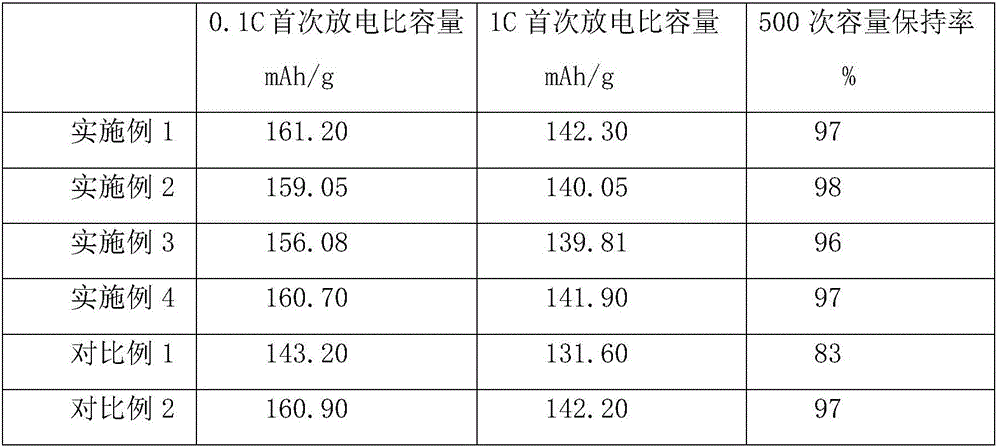
Embodiment 1
[0019] (1) Mix iron oxide and iron orthophosphate at a weight ratio of 1:1 to form an iron source, and mix the iron source, lithium dihydrogen phosphate, and lithium carbonate at a molar ratio of Li:P:Fe of 1:0.85:0.9 , and then mixed with 3% sucrose accounting for the total weight to obtain a mixture;
[0020] (2) Mix the mixture and alcohol at a weight ratio of 1:1, grind for 10 hours and dry at 80°C for 10 hours to obtain a lithium iron phosphate precursor;
[0021] (3) Stir and mix 1 mass part of nano-silica and water at a weight ratio of 1:1, then add 30 mass parts of the lithium iron phosphate precursor for mixing, and then add -0.05% of the weight of the lithium iron phosphate precursor Tartaric acid, after stirring for 5 hours, dried at 80°C to obtain lithium iron phosphate powder;
[0022] (4) Secondary sintering of the lithium iron phosphate powder under the protection of nitrogen; the first stage: put the lithium iron phosphate powder in a calciner with a furnace t...
Embodiment 2
[0025] (1) Mix iron oxide and iron orthophosphate at a weight ratio of 1:2 to form an iron source, and mix the iron source, lithium dihydrogen phosphate, and lithium carbonate at a Li:P:Fe molar ratio of 1:0.85:0.9 , and then mix with maltose accounting for 5% of the total weight to obtain a mixture;
[0026] (2) Mix the mixture and alcohol at a weight ratio of 1:1, grind for 10 hours and then dry at 10°C for 20 hours to obtain a lithium iron phosphate precursor;
[0027] (3) Stir and mix 1 mass part of nano-alumina and water at a weight ratio of 1:1, then add 30 mass parts of the lithium iron phosphate precursor for mixing, and then add 0.08% of the lithium iron phosphate precursor weight ratio Tartaric acid, after stirring for 8 hours, dried at 90°C to obtain lithium iron phosphate powder;
[0028] (4) Secondary sintering of the lithium iron phosphate powder under the protection of nitrogen; the first stage: put the lithium iron phosphate powder in a calciner with a furnace...
Embodiment 3
[0031] (1) Mix iron oxide and ferric orthophosphate at a weight ratio of 1:0.5 to form an iron source, and mix the iron source, lithium dihydrogen phosphate, and lithium carbonate at a Li:P:Fe molar ratio of 1:0.85:0.9 , and then mixed with 10% sucrose accounting for the total weight to obtain a mixture;
[0032] (2) Mix the mixture with alcohol at a weight ratio of 0.5:1, grind for 20 hours and then dry at 90°C for 15 hours to obtain a lithium iron phosphate precursor;
[0033] (3) Stir and mix 1 mass part of nano-silica sol and alcohol at a weight ratio of 2:1, then add 10 mass parts of lithium iron phosphate precursor for mixing, and then add 0.02 % tartaric acid, after stirring for 10 hours, dry at 100°C to obtain lithium iron phosphate powder;
[0034] (4) Secondary sintering of the lithium iron phosphate powder under the protection of nitrogen; the first stage: put the lithium iron phosphate powder in a calciner with a furnace temperature of 300°C for 4 hours and calcin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com