Membrane filtration, separation and purification method of levodopa conversion solution
A technology of membrane filtration separation and levodopa, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of long reaction time, high residual concentration of substrate, low crystallinity, etc., and achieve improvement The effect of moisture uniformity, shortening the production cycle, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 Tyrosine phenol lyase biocatalytic process
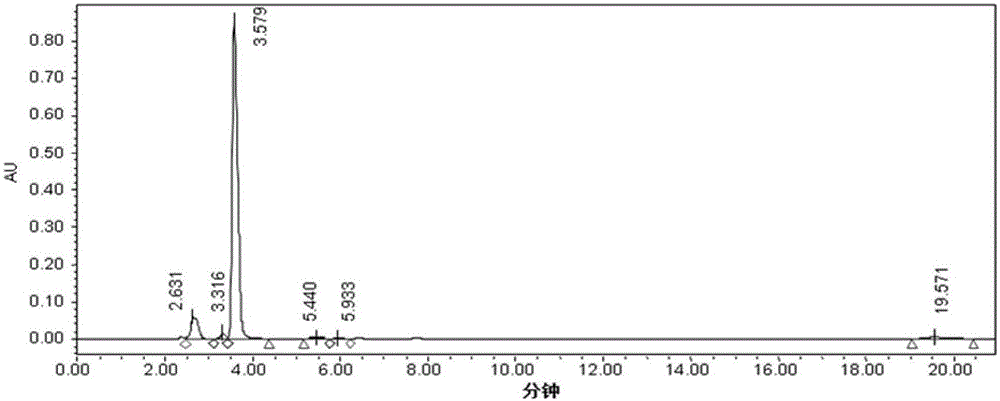
[0031]Put initial pyruvate into the water, control the pH at 7.0-9.0 with concentrated ammonia water; then add antioxidants, chelating agents, ammonium salts, pyridoxal 5-phosphate in sequence, and control the pH at 7.0-9.0 with concentrated ammonia water again, add The enzyme solution of recombinant tyrosine phenol lyase with a concentration of 80-200g / L is passed through nitrogen, the temperature is adjusted to the reaction temperature, the pH is adjusted to 7.0-9.0 with ammonia water, and catechol is added to start the reaction; the initial basic substrate o The molar ratio of hydroquinone to pyruvic acid and its sodium salt is 1:1-1.5. Afterwards, add the substrates catechol and pyruvate (or ammonium pyruvate, only pyruvate unless otherwise specified) at 2 g / L every 10 minutes, and the final concentration of catechol is 50-70 g / L (calculated based on the initial volume), the final concentration of pyruvic a...
Embodiment 2
[0036] Example 2 2500L transformation system
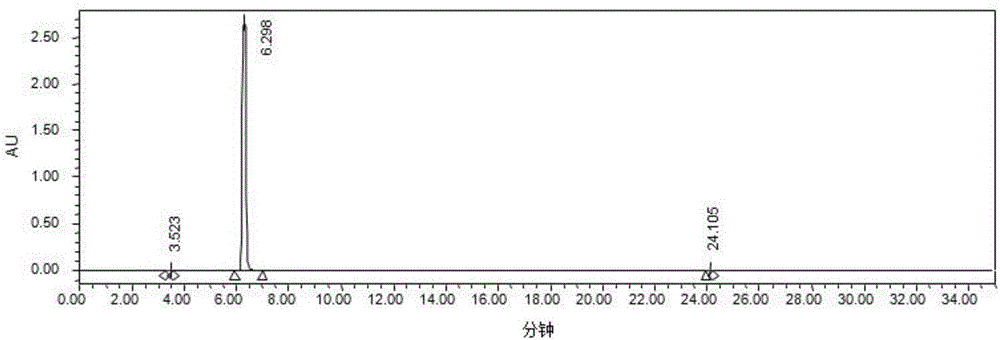
[0037] Take 2500 L of the levodopa conversion solution obtained in Example 1, with a concentration of 110 g / L, add concentrated hydrochloric acid to adjust the pH to 0.96, and the consumption of concentrated hydrochloric acid is 190 kg. The temperature of the feed liquid rises to 30-40°C, and the ceramic membrane system is turned on for filtration. The ceramic membrane system of Jiangsu Jiuwu High-tech Co., Ltd. is selected. The ceramic membrane element has a pore size of 50nm and is made of zirconium dioxide. A single membrane has 19 channels, and the total membrane area is 21.2 square meters.
[0038] Use the ceramic membrane to collect the supernatant, and when the minimum circulation volume is reached, about 500L system, start to add purified water. Detect the content of levodopa in the retentate, when down to 5g / L, stop filtering, the total volume of retentate is 530L. The collected supernatant has a volume of 3000 L, and t...
Embodiment 3
[0046] Example 3 5000L transformation system
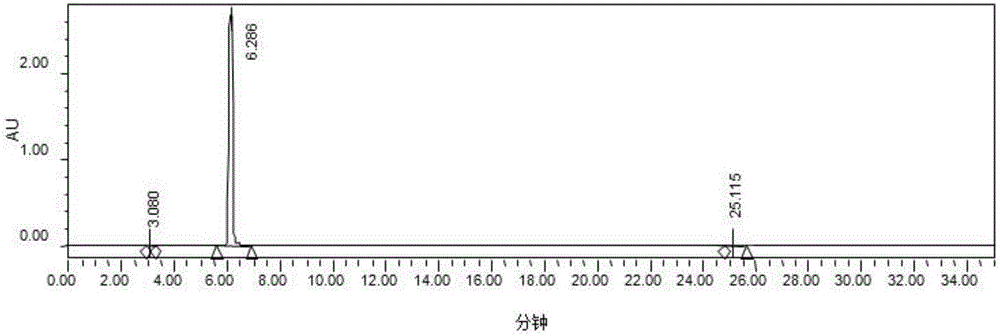
[0047] Take 5000L of the levodopa conversion liquid obtained in Example 1, the concentration is 115g / L, add concentrated nitric acid to adjust the pH to 1.04, the amount of concentrated nitric acid is 325kg, the temperature of the feed liquid is raised to between 30-40°C, and the ceramic membrane system is started for filtration. The ceramic membrane system of Jiangsu Jiuwu High-tech Co., Ltd. is selected. The ceramic membrane element has a pore size of 50nm and is made of zirconium dioxide. A single membrane has 19 channels, and the total membrane area is 21.2 square meters.
[0048] Use the ceramic membrane to collect the supernatant, and when the minimum circulation volume is reached, about 700L system, start to add drinking water. Detect the content of levodopa in the retentate, when down to 8g / L, stop filtering, the total volume of retentate is 830L. The volume of the collected supernatant is 6200L, and the content of levodo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com