Multi-armed polyglutamic acid taking beta-cyclodextrin as nucleus and injectable hydrogel and preparation method thereof
A polyglutamic acid, polyglutamic acid benzyl ester technology, applied in the field of injectable hydrogel and its preparation, multi-arm polyglutamic acid, can solve the problem of difficult hydrophobic drugs, etc., to achieve broad application prospects, good Effects of biodegradability and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
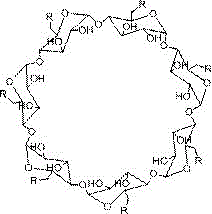
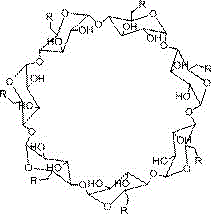
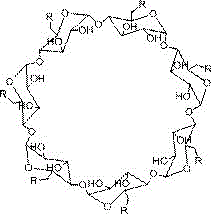
[0024] 1. Take a dry round-bottomed flask (100mL), add DMSO (20mL) and β-cyclodextrin (0.88 mmoL), stir well to dissolve, then add CDI (7.04mmoL), react under the protection of inert gas for 12~24h, Then add ethylenediamine (2.4mL) and continue to react for 12~24h. After pouring the reaction solution into excess absolute ethanol, a large amount of white matter is precipitated. The product is filtered and dried, dissolved in deionized water, and dialyzed with deionized water Freeze-drying can obtain about 0.5g of white product, which is polyamino β-cyclodextrin, and its structural formula is:
[0025]
[0026] Wherein the structural formula of R is:
[0027] .
[0028] 2. Put polyamino β-cyclodextrin (0.5g) in an anhydrous round bottom flask (100mL), add anhydrous DMSO (125mL), add L-glutamic acid-N-carboxylic acid anhydride ( 3.2g), vacuumize and blow nitrogen, and stir the reaction at room temperature for 72h. The reaction solution was poured into absolute ethanol (5...
Embodiment 2
[0044] The preparation of the aldylated multi-arm poly-L-glutamic acid in this example is basically the same as in Example 1, except that in step 2, polyamino β-cyclodextrin (1.0 g) was added, and DMSO was added (125mL), dissolved and added L-glutamic acid-N-carboxylic acid anhydride (3.2g). The multi-arm poly-L-glutamic acid finally obtained has an average molecular weight of about 2200 Daltons. The dosage and operating conditions of other reagents remained unchanged. The finally obtained injectable hydrogel had a gelation time of 81 s and a storage modulus of 81 Pa.
Embodiment 3
[0046] The preparation of the aldylated multi-arm poly-L-glutamic acid in this example is basically the same as that in Example 1, except that in step 2, polyamino β-cyclodextrin (0.25 g) was added and DMSO was added (125mL), dissolved and added L-glutamic acid-N-carboxylic acid anhydride (3.2g). The multi-arm poly-L-glutamic acid finally obtained has an average molecular weight of about 11,000 Daltons. The dosage and operating conditions of other reagents remained unchanged. The finally obtained injectable hydrogel had a gelation time of 86s and a storage modulus of 196Pa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com