Method for detecting carbendazim content in edible fungi by using carbendazim-specific antibody
A technology for antibody detection and carbendazim, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that have not been reported in the literature, achieve high yield, simple sample pretreatment steps, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of carbendazim hapten
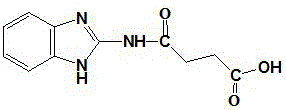
[0032] Stir and dissolve 0.5g of 2-aminobenzimidazole in 100mL of acetonitrile in a 250mL beaker, then add 0.564g of succinic anhydride, stir and react at 40°C for 4 hours, a large amount of white precipitate precipitates, filter the reaction solution to obtain a filter residue, and filter the residue Rinse twice with acetonitrile at 40°C to remove unreacted raw materials remaining in the filter residue to obtain a crude product. The crude product was further purified by column chromatography (mobile phase was ethyl acetate:n-hexane=1:3, v / v), and identified by thin layer chromatography and mass spectrometry, the obtained product was 2-succinylbenzimidazole (white powder), its structural analysis see figure 2 .
[0033] (2) Preparation of carbendazim immunogen
[0034]In a 4 mL brown vial, stir and dissolve 18.6 mg hapten in 0.9 mL N,N-dimethylformamide (DMF), then add dropwise a mixed solution of 13.8 mg NHS and 24.7 mg DCC dis...
Embodiment 2
[0040] 1. Establish the standard curve of carbendazim by direct competition ELISA method:
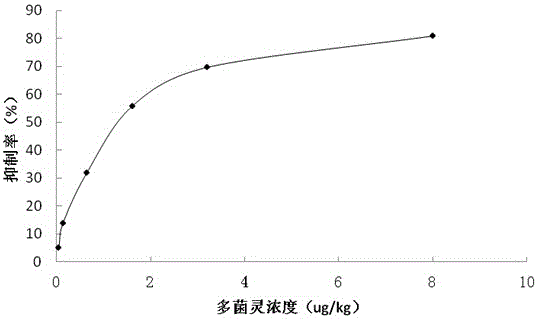
[0041] The optimal coating volume of the antibody was 5ug / mL, the optimal dilution factor of the enzyme-labeled antigen was 150 times, and the optimal ion concentration and pH value were 0.01MPBS with pH=7.4. Using the optimized experimental conditions, the standard curve is established with the standard concentration (ug / kg) as the abscissa and the inhibition rate (%) as the ordinate. The results are shown in figure 1 ,Specific steps are as follows:
[0042] (1) Coating: Prepare the antibody with 0.01M carbonic acid buffer (pH=9.6) to make a 5ug / mL coating solution, add 100uL coating solution to each well of the microplate plate, incubate at 4°C for 12h, shake dry, and use Wash 3 times with PBST;
[0043] (2) Adding samples: add 100uLPBS to the blank well; add 50uLPBS and 50uL enzyme-labeled antigen diluted 150 times to the control well; add 50uL standard products of different concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com