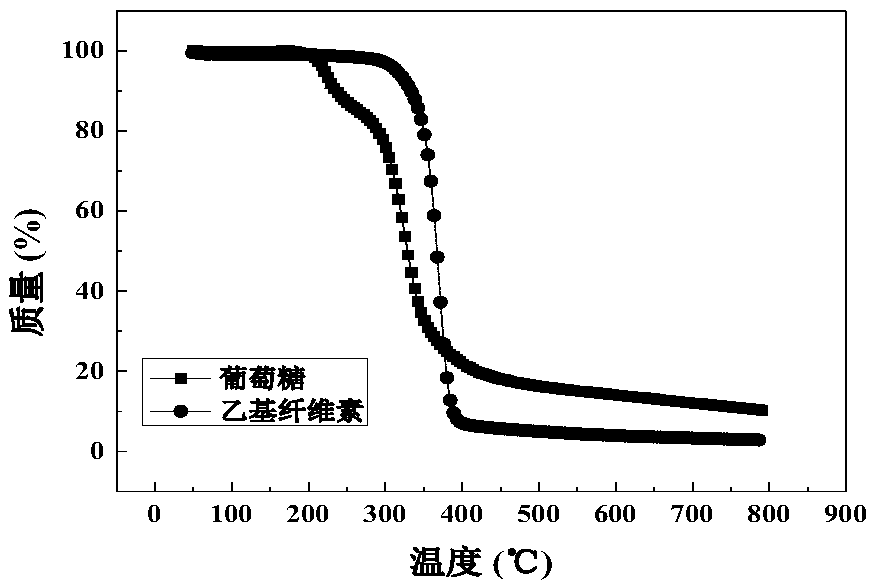
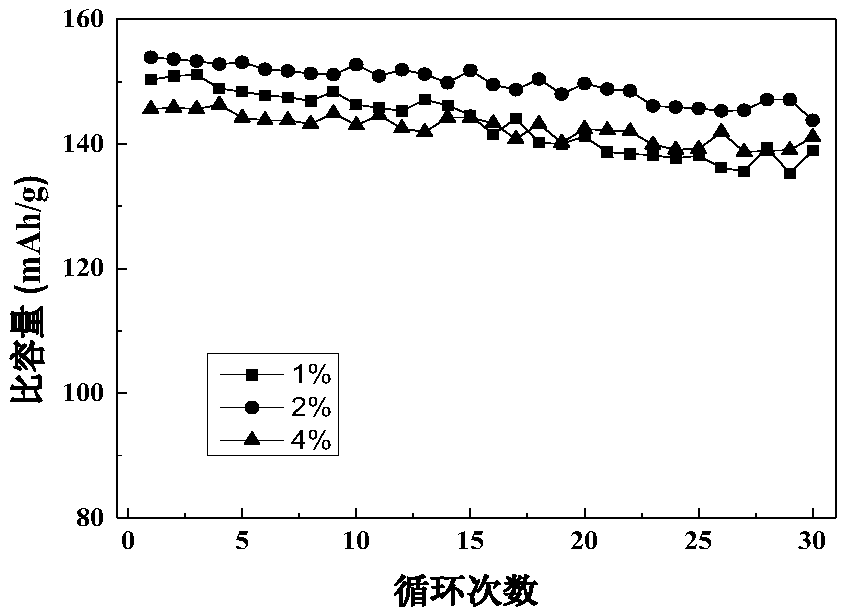
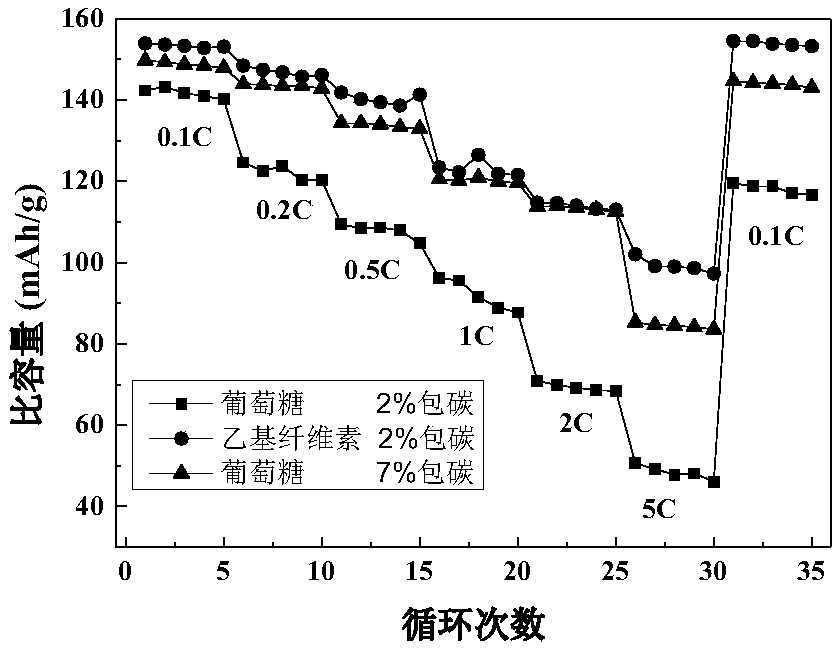
A method for preparing lithium manganese iron phosphate positive electrode material using ethyl cellulose as carbon source
A technology of lithium manganese iron phosphate and ethyl cellulose, which is applied in the direction of chemical instruments and methods, battery electrodes, phosphorus compounds, etc., can solve the problems of affecting the battery capacity and the reduction of the gram capacity of the synthetic product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] ① Manganous sulfate (0.0384mol), ferrous sulfate (0.0096mol), phosphoric acid (0.048mol), ascorbic acid (0.014mol) (that is, according to LiMn X Fe 1-X PO 4 (X = 0.8) for weighing) dissolved in 160ml of water and ethylene glycol by volume ratio = 1:2 in the mixed solvent composition, called A liquid; dissolved lithium hydroxide (0.144mol) in 160ml of water and ethylene glycol Alcohol in the mixed solvent composed of volume ratio = 1:2 in solvent two, called B liquid. Then, liquid B was added dropwise to liquid A for 20 minutes to obtain a precursor solution of lithium manganese iron phosphate, wherein the total concentration of ascorbic acid in the precursor solution was 0.0438 mol / L, and the lithium ion concentration in the precursor solution was 0.45 mol / L. Place it in a high-temperature and high-pressure reactor, the volume of the precursor solution is 80% of the volume of the reactor, heat it to 240°C, and the reaction time is 4h. ℃, -0.1MPa vacuum drying to prep...
Embodiment 2
[0047] ① Manganous sulfate (0.0096mol), ferrous sulfate (0.0384mol), phosphoric acid (0.048mol), ascorbic acid (0.024mol), glucose (0.024mol) (that is, according to LiMn X Fe 1-X PO 4 (X = 0.2) for weighing) dissolved in 200ml of water and ethylene glycol by volume ratio = 1:1 mixed solvent, called A liquid; dissolved lithium hydroxide (0.1548mol) in 120ml of water and ethylene glycol Alcohol in the mixed solvent composed of volume ratio = 1:1 in solvent two, called B liquid. Then add liquid B dropwise to liquid A for 15 minutes to obtain a precursor solution of lithium manganese iron phosphate, wherein the total concentration of ascorbic acid and glucose in the precursor solution is 0.15mol / L, and the lithium ion concentration in the precursor solution is 0.4838mol / L . Place it in a high-temperature and high-pressure reactor, the volume of the precursor solution is 40% of the volume of the reactor, heat to 200°C, and the reaction time is 4h. ℃, -0.1MPa vacuum drying to pr...
Embodiment 3
[0051] The reaction equation in the present embodiment hydrothermal process is:
[0052] 3LiOH+XMn(NO 3 ) 2 +(1-X)Fe(NO 3 ) 2 +H 3 PO 4 →LiMn x Fe 1-x PO 4 +2LiNO 3 +3H 2 o
[0053] where X=0.1-0.9;
[0054] The specific experiment is:
[0055] ① Manganous nitrate (0.0336mol), ferrous nitrate (0.0144mol), phosphoric acid (0.048mol), ascorbic acid (0.0096mol) (that is, according to LiMn X Fe 1-X PO 4 (X = 0.7) for weighing) dissolved in 160ml of water and ethylene glycol by volume ratio = 1:2 in the mixed solvent, called A liquid; dissolved lithium hydroxide (0.144mol) in 120ml of water and ethylene glycol Alcohol in the mixed solvent composed of volume ratio = 1:2 in solvent two, called B liquid. Then, liquid B was added dropwise to liquid A for 20 minutes to obtain a precursor solution of lithium manganese iron phosphate, wherein the total concentration of ascorbic acid in the precursor solution was 0.0343 mol / L, and the lithium ion concentration in the precur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com