Separation and purification method for monoclonal antibody
A monoclonal antibody, separation and purification technology, applied in the preparation methods of peptides, chemical instruments and methods, anti-animal/human immunoglobulins, etc. The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
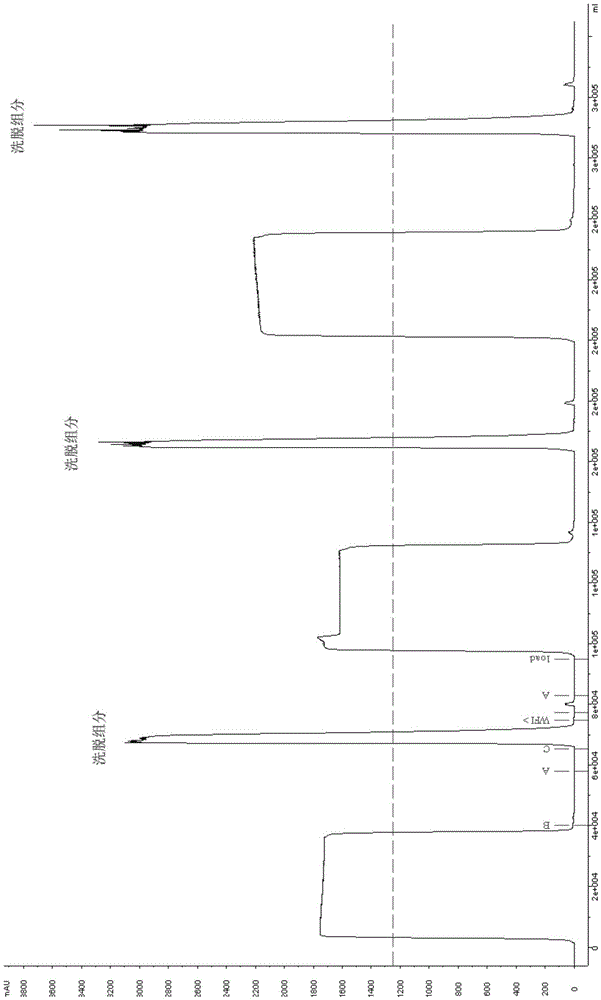
[0044] The cell culture supernatant harvested from the 150L fermenter was divided into three parts, and the Mabselect SuRe chromatography column was used for the first step of purification three times, and 40L of the first sample was loaded on the equilibrated Mabselect SuRe chromatography column (BPG200 / 50) In, after loading the sample, rinse the chromatography column with buffer A (containing 20mM PB, 150mM NaCl, pH 7.2), and then wash the column with buffer B (containing 20mM PB, 1M NaCl, pH 6.5) and buffer A ( Contains 20mM PB, 150mM NaCl, pH 7.2) to wash the chromatographic column, then elute with buffer C (ie 0.1M NaAC-HAc, pH 3.4), collect the eluted fraction; repeat this purification step twice, the purification process chromatogram picture see figure 1 ; Each collected elution fraction (pH 3.7, referred to as "elution fraction I") was left at room temperature for 2 hours, then diluted with pH 7.2 phosphate buffer to neutralize to pH 5.2; three dilutions were combined ...
Embodiment 2
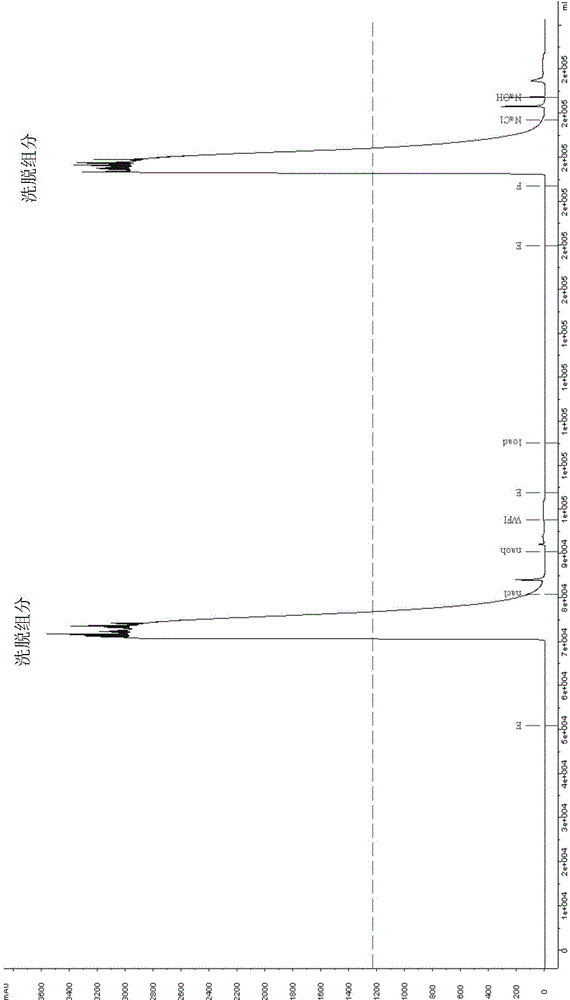
[0046] Load the cell culture supernatant harvested from the 7.5L fermenter on the equilibrated Mabselect SuRe LX chromatographic column (XK50 / 30). ) to wash the chromatography column, then wash the chromatography column with buffer B (containing 20mM PB, 1M NaCl, pH 7.0) and buffer A (containing 20mM PB, 120mM NaCl, pH 7.5) in sequence, and then wash the chromatography column with buffer C ( That is, 0.1M glycine, pH 3.2) was eluted, and the eluted fraction was collected, called eluted fraction I; the eluted fraction I (pH 3.5) was left at room temperature for 1 hour, and then diluted with phosphate buffer solution of pH 7.2 Neutralize (to pH 5.5), load the sample on the equilibrated POROS XS chromatography column (XK50 / 20), after loading, wash the column with buffer D (20mM PB, pH 6.2), and then use buffer Solution E (20mM PB, 100mMNaCl, pH 6.2) was eluted, and the eluted fraction was collected, called eluted fraction II; the eluted fraction II was diluted 2 times with 20mM P...
Embodiment 3
[0048] Load the cell culture supernatant harvested from the 7.5L fermenter on the equilibrated ProSep Ultra Plus chromatography column. After loading, wash the chromatography with buffer A (including 20mM PB, 180mM NaCl, pH 7.0) Column, then wash the column with buffer B (containing 20mM PB, 1.5M NaCl, pH 6.0) and buffer A (containing 20mM PB, 180mM NaCl, pH 7.0) in sequence, and then wash the chromatography column with buffer C (that is, 0.1M glycine , pH 3.0) elution, collect the elution fraction, called elution fraction I; leave the elution fraction I (pH 3.2) at room temperature for 0.5 hours, then dilute and neutralize to pH 5 with pH 8.0 phosphate buffer .8. Load the sample on the equilibrated Eshmuno CPX chromatography column. After loading the sample, wash the column with buffer D (20mM PB, 50mM NaCl, pH 5.8), and then wash the column with buffer E (20mM PB, 200mM NaCl , pH 5.8) elution, collect the elution fraction, called elution fraction II; dilute the elution fract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com