Protein transduction peptide-paraoxonase 1 fusion protein and preparation method and application thereof
A technology of protein transduction peptide and fusion protein, which is applied in the field of fusion protein of protein transduction peptide and paraoxonase 1, and can solve the problems of reducing curative effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
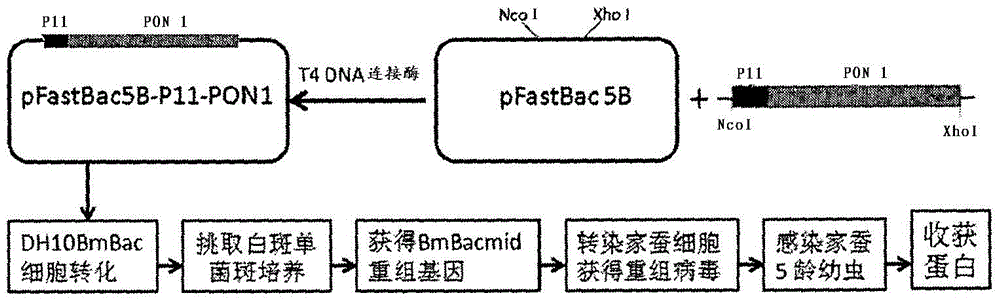
[0061] Embodiment 1. Bombyx mori expression vector construction and silkworm expression
[0062] The artificially synthesized P11-PON1 fusion gene NCOI+PTD+PON1+XhoI sequence is as follows:
[0063] CCATGGGC TATGGTCGTAAAAAACGTCGCCAGCGTCGCCGTAtggcgaagctgattgcgctcaccctcttggggatgggactggcactcttcaggaaccaccagtcttcttaccaaacacgacttaatgctctccgagaggtacaacccgtagaacttcctaactgtaatttagttaaaggaatcgaaactggctctgaagac ttg gagatactgcctaatggactggctttcattagctctggattaaagtatcctggaataaagagcttcaaccccaacagtcctggaaaaatacttctgatggacctgaatgaagaagatccaacagtgttggaattggggatcactggaagtaaatttgatgtatcttcatttaaccctcatgggattagcacattcacagatgaagataatgccatgtacctcctggtggtgaaccatccagatgccaagtccacagtggagttgtttaaatttcaagaagaagaaaaatcgcttttgcatctaaaaaccatcagacataaacttctgcctaatttgaatgatattgttgctgtgggacctgagcacttttatggcacaaatgatcactattttcttgacccctactta caa tcctgggagatgtatttgggtttagcgtggtcgtatgttgtctactatagtccaagtgaagttcgagtggtggcagaaggatttgattttgctaatggaatcaacatttcacccgatggcaagtatgtctatatagctgagttgctggctcataagattcatgtgt...
Embodiment 2
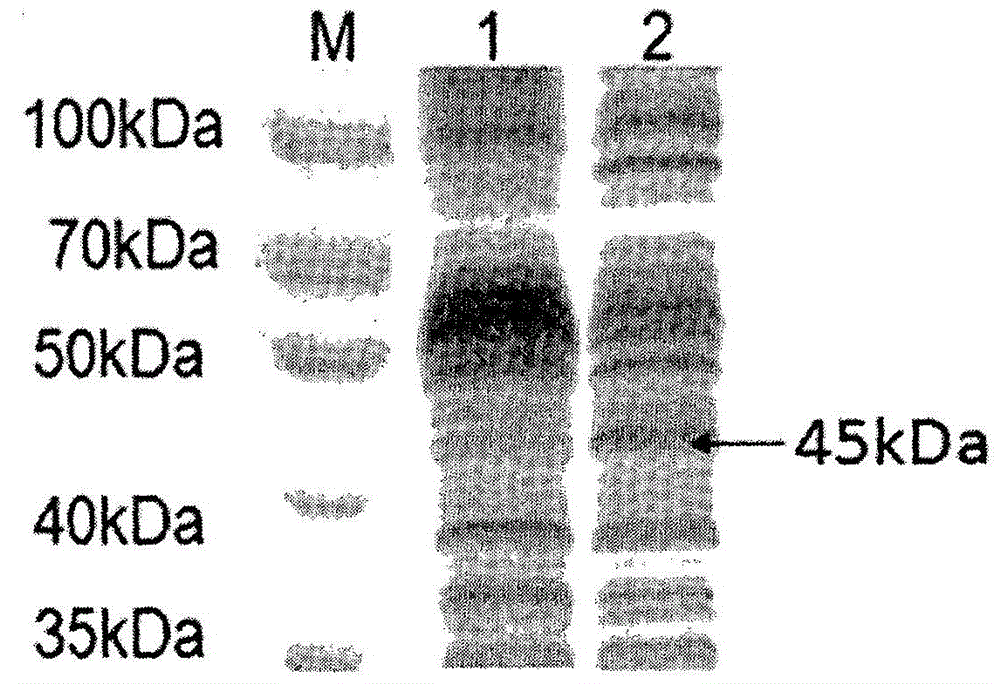
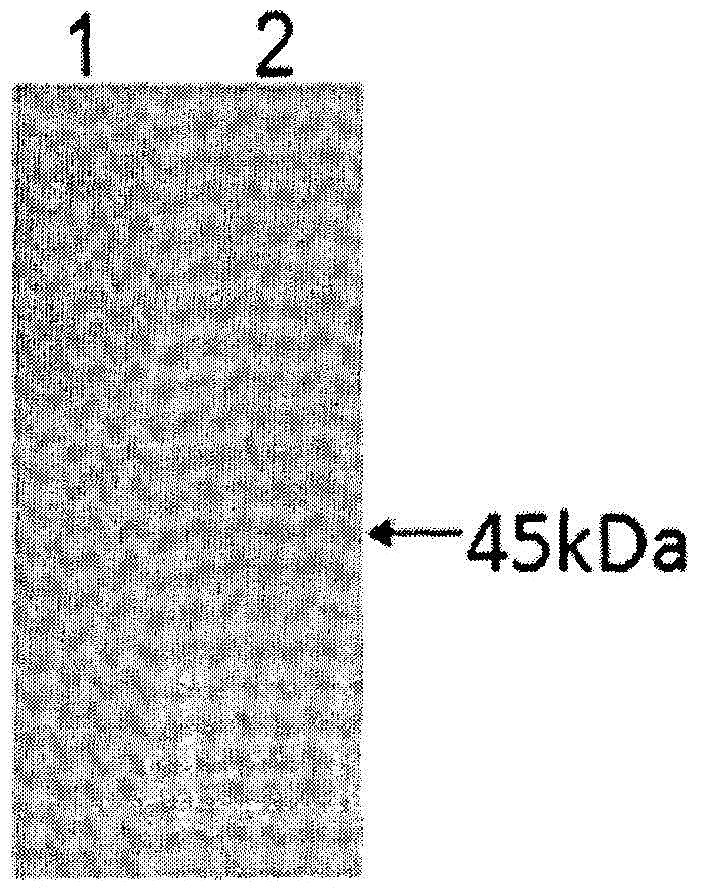
[0067] Example 2 Identification of P11-PON1 fusion protein
[0068] Take 1 gram of dry insect body powder, dissolve it in 20ml of PBS solution, sonicate at 4°C for 20 minutes, centrifuge at 12,000r / min for 10 minutes, collect the supernatant, and use the BCA protein quantification kit to determine the total amount of silkworm protein; take 10 μl of the supernatant for SDS-PAGE For electrophoresis, prepare two 12% SDS-PAGE gels. After electrophoresis, one piece is stained with Coomassie brilliant blue for 1 hour, decolorized with glacial acetic acid overnight, and the Gel-pro gel imaging system is used to take pictures and scan to calculate the proportion of P11-PON1 fusion protein in the total Protein ratio, and finally calculate the net content of P11-PON1 fusion protein; another piece of gel is used for Western blot identification, 37 ° C after transfer, 5% skimmed milk blocking 2h, primary antibody (rabbit anti-human PON1 polyclonal antibody diluted 1:1000 ) at 4°C overnigh...
Embodiment 3
[0069] Example 3 The biological activity of P11-PON1 fusion protein
[0070] 3.1 Biological activity of P11-PON1 fusion protein on mice
[0071] Kunming mice were half male and half male, weighing 25±3 grams, fasted for 12 hours before the experiment to empty the stomach and intestines as much as possible, and drink water freely. The experimental groups were divided into intragastric administration group and rectal administration group, and the control group was intraperitoneally injected with 20 mg / kg body weight of dichlorvos. The experiment was repeated 3 times, with 6 rats in each group, half male and half male. Each mouse in the gavage group was directly given the expression supernatant (P11-PON1 fusion protein and PON1 protein (net content of PON1 protein 1 mg)) through the gavage needle; It tries to empty the feces in the rectum as much as possible, then probes into about 1.5cm with a gastric gavage needle, injects slowly, and withdraws the needle slowly after stoppin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com