Sustained release preparation used for treating alzheimer's disease, and preparation method thereof
A technology of sustained-release preparations and sustained-release materials, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and pill delivery, etc. smooth and complete effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
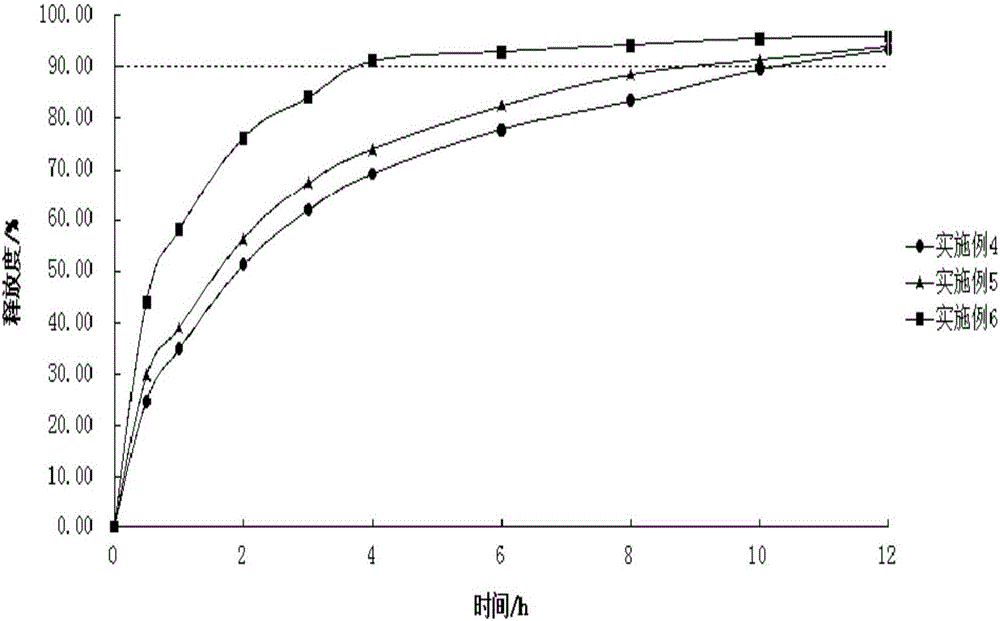
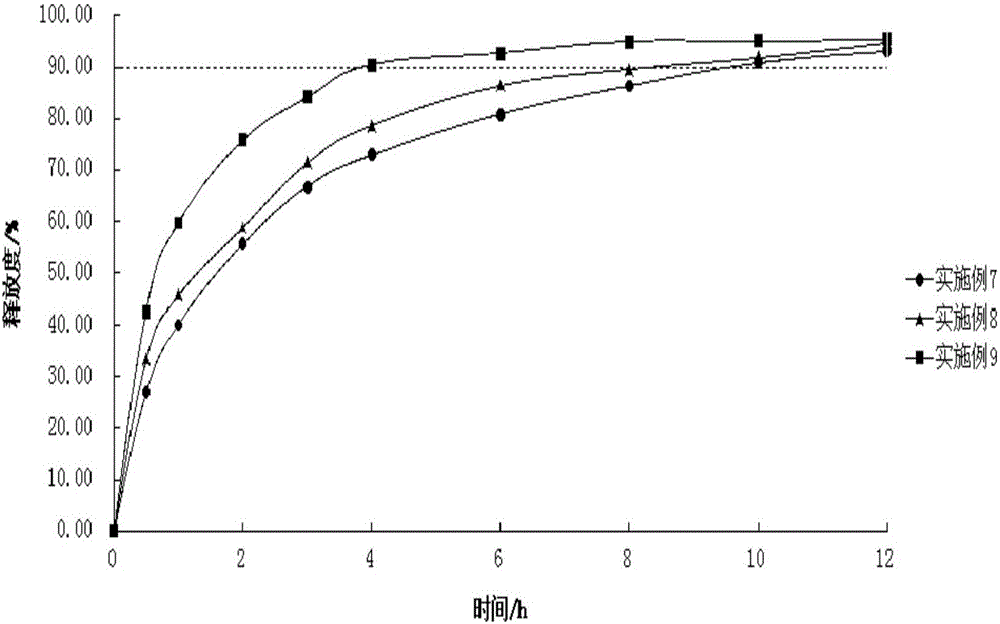
Examples
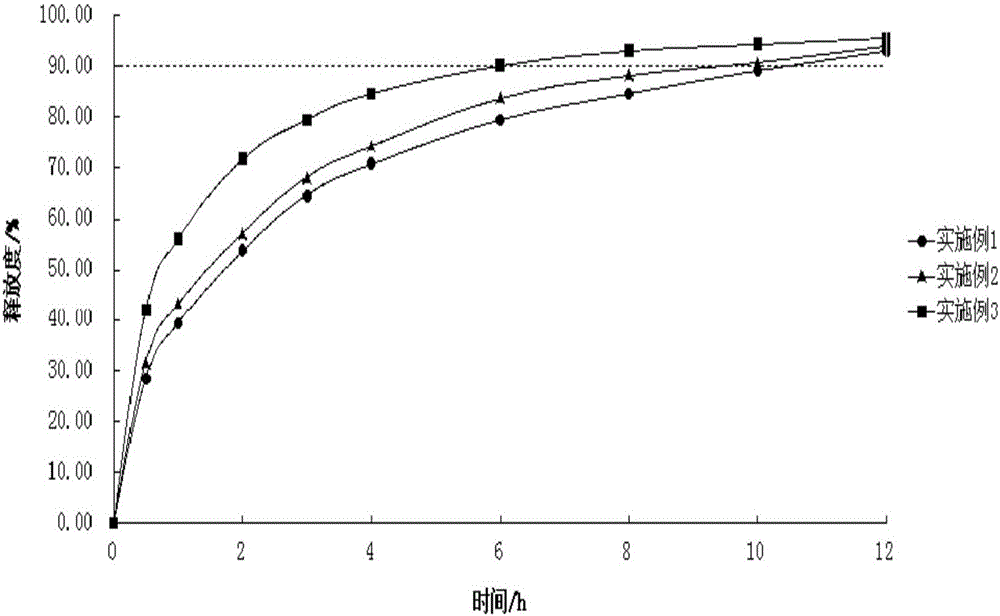
Embodiment 1
[0025] Example 1: The formulation of the sustained-release formulation of 2-(4-methylthiazol-5-yl)ethyl nitrate hydrochloride is as follows:
[0026]
[0027] Taking the preparation of 10,000 2-(4-methylthiazol-5-yl)ethyl nitrate hydrochloride sustained-release preparations as an example, all the excipients were crushed separately and passed through a 100-mesh nylon sieve. Take the main medicine and grind to powder. According to the prescription composition in Example 1, 441.0g of 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride, 450.0g of hydroxypropyl methylcellulose K100M, 417.0 lactose, 139.5g micro Crystal cellulose PH101, mix well, make soft material with 4% povidone ethanol solution, granulate with 20-mesh sieve, dry at 50°C, granulate with 18-mesh sieve, add 7.5g magnesium stearate and 45.0g dioxide Silicon, mix well, press with φ7 punch. The 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride sustained-release preparation is obtained. The release rate was determi...
Embodiment 2
[0028] Example 2: The formulation of the sustained-release formulation of 2-(4-methylthiazol-5-yl)ethyl nitrate hydrochloride is as follows:
[0029]
[0030] Taking the preparation of 10,000 2-(4-methylthiazol-5-yl)ethyl nitrate hydrochloride sustained-release preparations as an example, all the excipients were crushed separately and passed through a 100-mesh nylon sieve. Take the main medicine and grind to powder. According to the prescription composition in Example 2, 441.0g of 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride, 300.0g of hydroxypropyl methylcellulose K100M, 529.5g of lactose, and 177.0g were respectively weighed out. Microcrystalline cellulose PH101, mix well, make soft material with 4% povidone ethanol solution, granulate with 20 mesh sieve, dry at 50°C, granulate with 18 mesh sieve, add 7.5g magnesium stearate and 45.0g sodium bicarbonate Silicon oxide, mix well, press with φ7 punch. The 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride sustained-rel...
Embodiment 3
[0031] Example 3: The formulation of the sustained-release formulation of 2-(4-methylthiazol-5-yl)ethyl nitrate hydrochloride is as follows:
[0032]
[0033]
[0034] Taking the preparation of 10,000 2-(4-methylthiazol-5-yl)ethyl nitrate hydrochloride sustained-release preparations as an example, all the excipients were crushed separately and passed through a 100-mesh nylon sieve. Take the main medicine and grind to powder. According to the prescription composition in Example 3, 441.0g of 2-(4-methylthiazol-5-yl)ethyl nitrate hydrochloride, 150.0g of hydroxypropyl methylcellulose K100M, 642.0g of lactose, and 214.5g were respectively weighed out. Microcrystalline cellulose PH101, mix well, make soft material with 4% povidone ethanol solution, granulate with 20 mesh sieve, dry at 50°C, granulate with 18 mesh sieve, add 7.5g magnesium stearate and 45.0g sodium bicarbonate Silicon oxide, mix well, press with φ7 punch. The 2-(4-methylthiazol-5-yl) ethyl nitrate hydrochloride susta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com