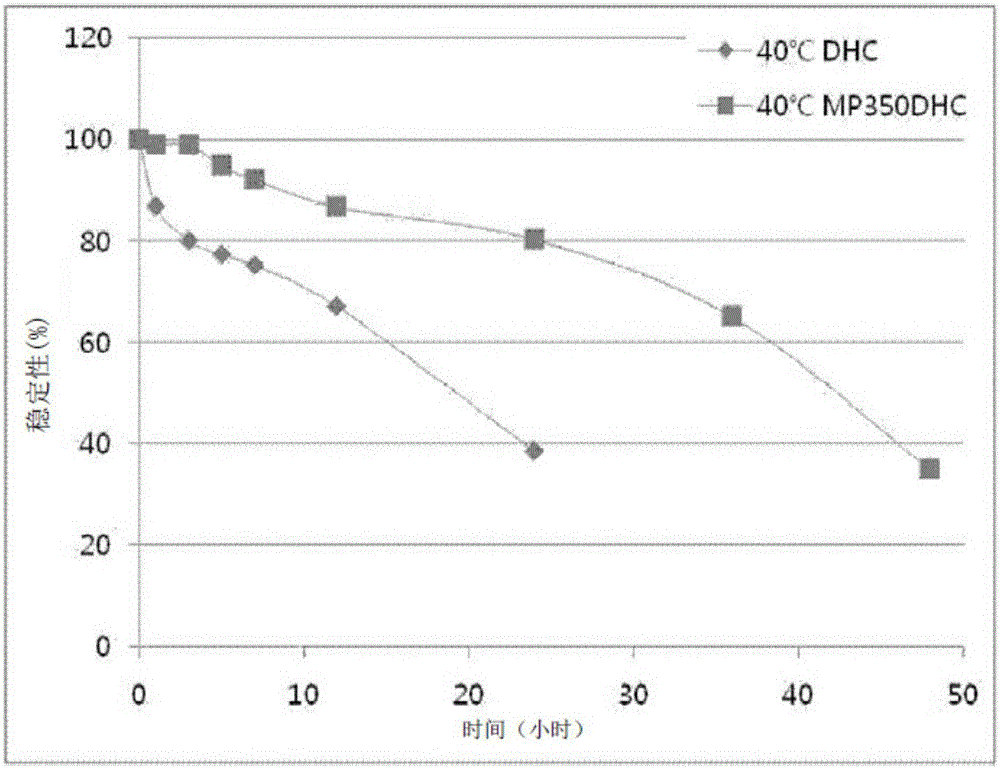
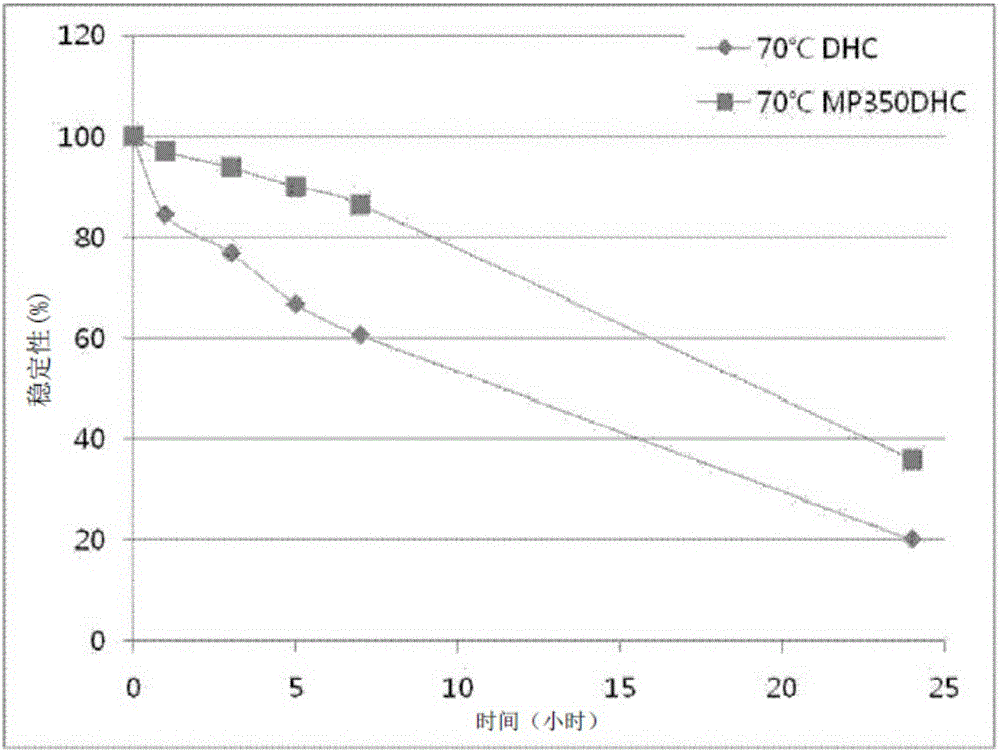
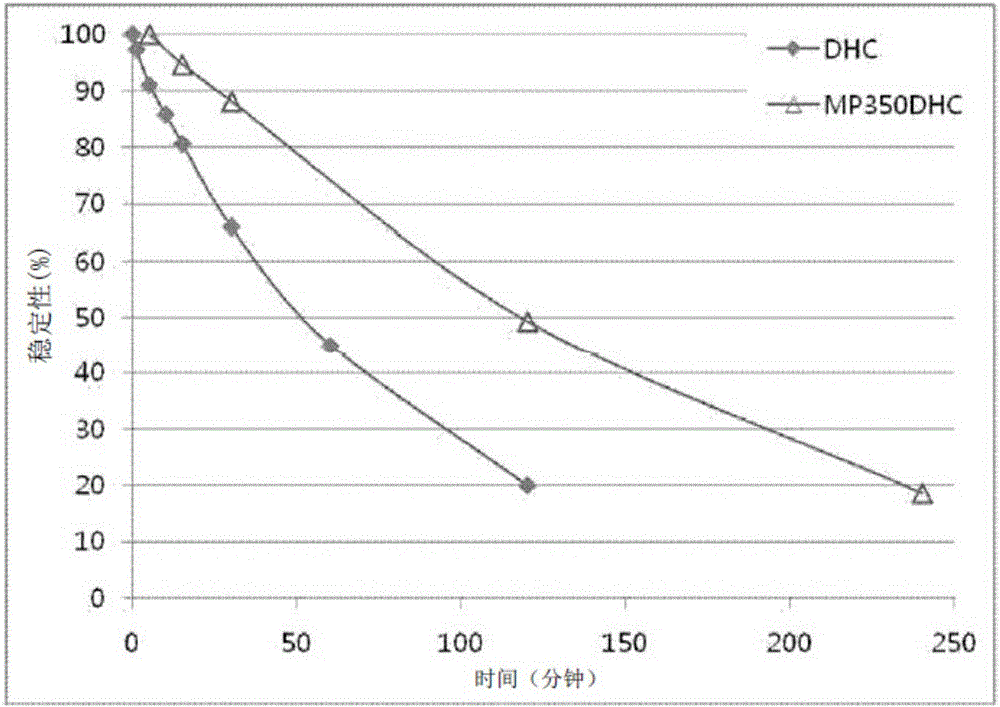
Pegylated 7-dehydrocholesterol derivative
A technology of PEGylation and dehydrocholesterol, applied in steroids, cosmetics, food science, etc., can solve the stagnation of 7-dehydrocholesterol research, structural denaturation, and no stability involved in the synthesis of 7-dehydrocholesterol sexual issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Example 1: Preparation of mPEG350-S-DHC ester (compound of formula (1A), wherein R is methyl, X is O, n is 7.25 (average value), and m is 1)
Embodiment 1-1
[0153] Example 1-1: Preparation of 7-dehydrocholesterol succinate (compound of formula (5), wherein m is 1)
[0154]Dissolve 7-dehydrocholesterol (DHC) (6.45g, 16.8mmol) (6.45g, 16.8mmol) of chemical formula (4) in 120ml dichloromethane, wherein add triethylamine (3.39g, 33.5mmol) and 4-dimethylaminopyridine (0.82g , 6.7mmol) to obtain a solution. Therein, succinic anhydride (compound of formula (3), wherein m is 1) (3.35 g, 33.5 mmol) was added, followed by stirring at room temperature for 12 hours. The reaction solution was washed successively with 3.5% HCl aqueous solution, saturated sodium bicarbonate aqueous solution and saturated sodium chloride aqueous solution. Then, the organic phase was dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (ethyl acetate:hexane=1:2) to obtain 7.2 g of the target compound.
[0155] 1 H NMR 400MHz (CDCl 3 )δ5.59(dd,1H),5.41(dd,1H),4.75...
Embodiment 1-2
[0156] Example 1-2: Preparation of mPEG350-S-DHC ester (compound of chemical formula (1A), wherein R is methyl, X is O, n is 7.25 (average value), and m is 1)
[0157] Dissolve the 7-dehydrocholesterol succinate of embodiment 1-1 (the compound of chemical formula (5), wherein m is 1) (1.1g, 2.30mmol) and mPEG350-OH (chemical formula (6) in 10ml of dichloromethane A compound wherein R is methyl, n is 7.25 (mean), and X is O) (0.8 g, 2.30 mmol), to which 4-dimethylaminopyridine (0.42 g, 3.44 mmol) was added. To this, N-(3-dimethylaminopropyl)-N'-ethyl-carbodiimide (0.88 g, 4.59 mmol) was slowly added, followed by stirring at room temperature for 12 hours. The reaction solution was washed successively with 1.0N aqueous HCl solution, saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution. Then, the organic phase was dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure, and dried under vacuum to obtain 1.77 g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com