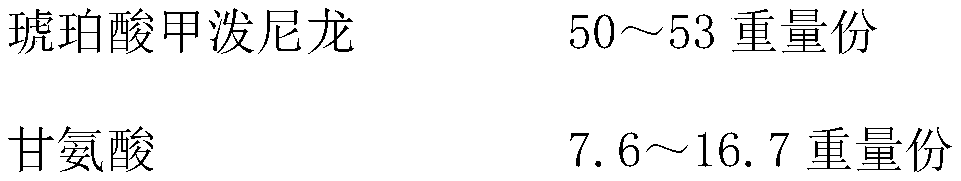A kind of methylprednisolone sodium succinate composition for injection
A technology of methylprednisolone sodium succinate and methylprednisolone succinate, which is applied in the field of medicine, can solve the problems of reducing visible precipitates, limited solubilization effect, and unproposed stability, so as to achieve drug safety and facilitate industrial production and preparation The effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0040] The preparation method of embodiment 1-5:
[0041] (1) Take by weighing sodium dihydrogen phosphate and disodium hydrogen phosphate of prescription quantity, add 100ml water for injection, make buffer solution, boil 20min, drop to room temperature, standby;
[0042] (2) Take by weighing the sodium bicarbonate of recipe quantity, add 50ml water for injection, stir and dissolve, boil 20min, be down to room temperature, standby;
[0043] (3) Weigh the prescribed amount of glycine and hydroxypropyl-β-cyclodextrin (substitution degree is 3-7), add 350ml water for injection, stir to dissolve, boil for 20min, drop to room temperature, and set aside;
[0044] (4) Add 300ml of water for injection to the prescribed amount (in terms of methylprednisolone) methylprednisolone succinate, and stir into a paste;
[0045] (5) Slowly add the step (2) solution into the step (4), and stir while adding, until the methylprednisolone succinate is completely dissolved;
[0046] (6) Add the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com