N,N-diamide substituted hydrazone derivative and synthesis method
A technology of diamide group and synthesis method, applied in the field of N,N-diamide group substituted hydrazone derivatives and synthesis, can solve problems such as lack of efficient synthesis, achieve good industrial application prospects, mild reaction conditions, no water and air sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
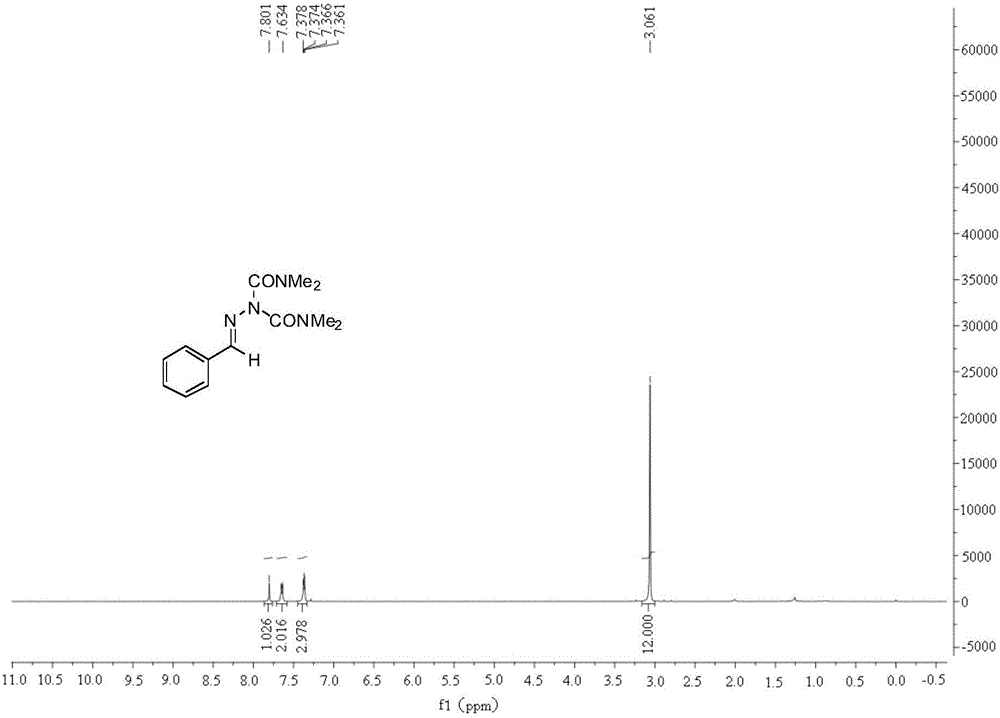
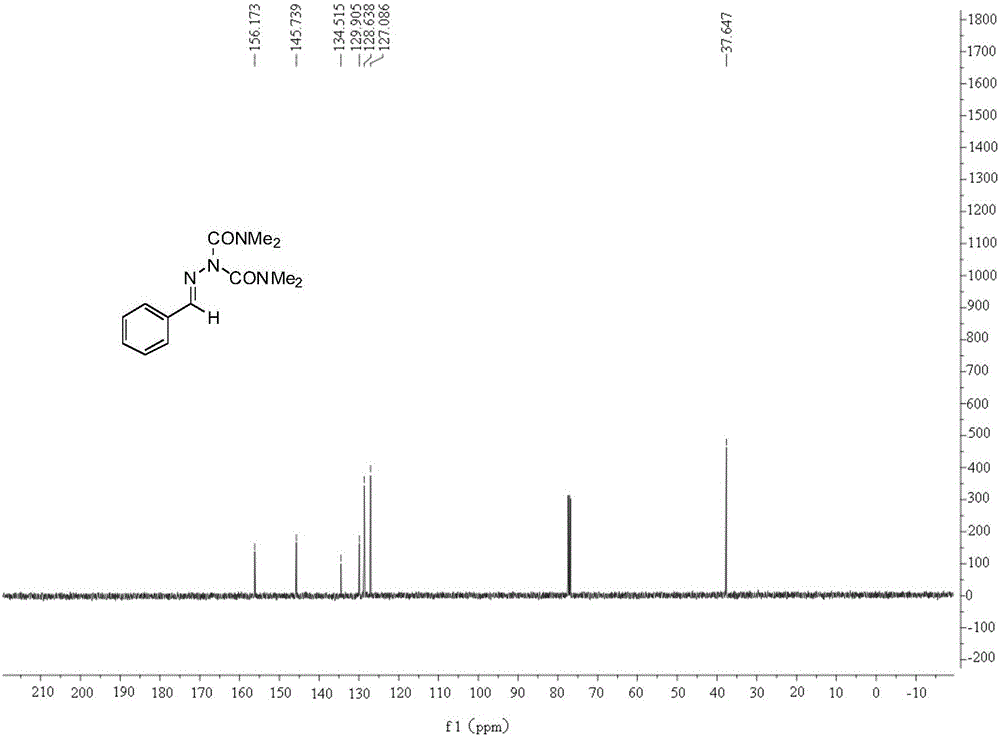
[0026] In a 25 ml reaction flask equipped with a reflux condenser, add 0.2 mmol of benzaldehyde-p-toluenesulfonyl hydrazone, 0.2 mmol of cesium carbonate, 0.01 mmol of palladium trifluoroacetate, 0.1 mmol of azodimethylformamide, 2 ml of toluene, the reaction system was stirred at 90° C. for 12 hours, the heating and stirring were stopped, and the mixture was cooled to room temperature. The reaction solution was extracted with ethyl acetate, the solvent was removed by rotary evaporation under reduced pressure, and the target product was obtained by separation and purification by column chromatography. The column chromatography eluent used was petroleum ether with a volume ratio of 2:1: ethyl acetate mixed solvent , the product was an oily liquid with a yield of 91%.
Embodiment 2
[0028] In a 25 ml reaction flask equipped with a reflux condenser, add 0.2 mmol of benzaldehyde-p-toluenesulfonyl hydrazone, 0.3 mmol of potassium carbonate, 0.01 mmol of palladium trifluoroacetate, 0.1 mmol of azodimethylformamide, 2 ml of toluene, the reaction system was stirred at 90° C. for 12 hours, the heating and stirring were stopped, and the mixture was cooled to room temperature. The reaction solution was extracted with ethyl acetate, the solvent was removed by rotary evaporation under reduced pressure, and the target product was obtained by separation and purification by column chromatography. The column chromatography eluent used was petroleum ether with a volume ratio of 2:1: ethyl acetate mixed solvent , the product was an oily liquid with a yield of 83%.
Embodiment 3
[0030] In a 25 ml reaction flask equipped with a reflux condenser, add 0.2 mmol of benzaldehyde-p-toluenesulfonyl hydrazone, 0.2 mmol of potassium tert-butoxide, 0.01 mmol of palladium trifluoroacetate, 0.1 mmol of azodimethylformamide , 2 ml of toluene, the reaction system was stirred at 90 ° C for 12 hours, the heating and stirring were stopped, and it was cooled to room temperature. The reaction solution was extracted with ethyl acetate, the solvent was removed by rotary evaporation under reduced pressure, and the target product was obtained by separation and purification by column chromatography. The column chromatography eluent used was petroleum ether with a volume ratio of 2:1: ethyl acetate mixed solvent , the product was an oily liquid with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com