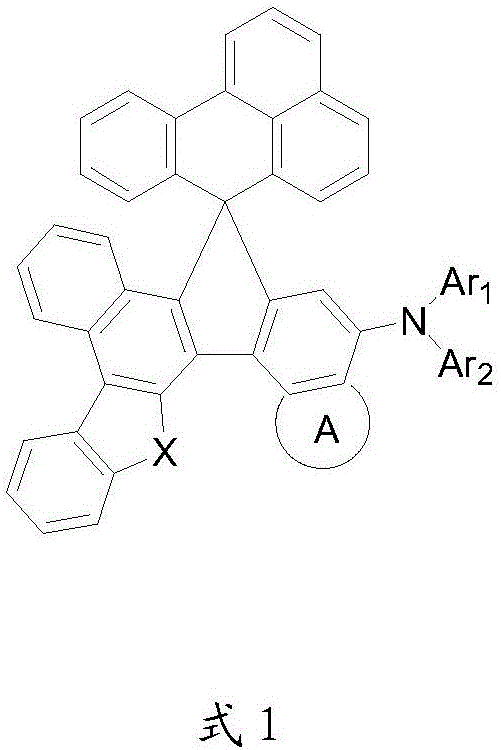
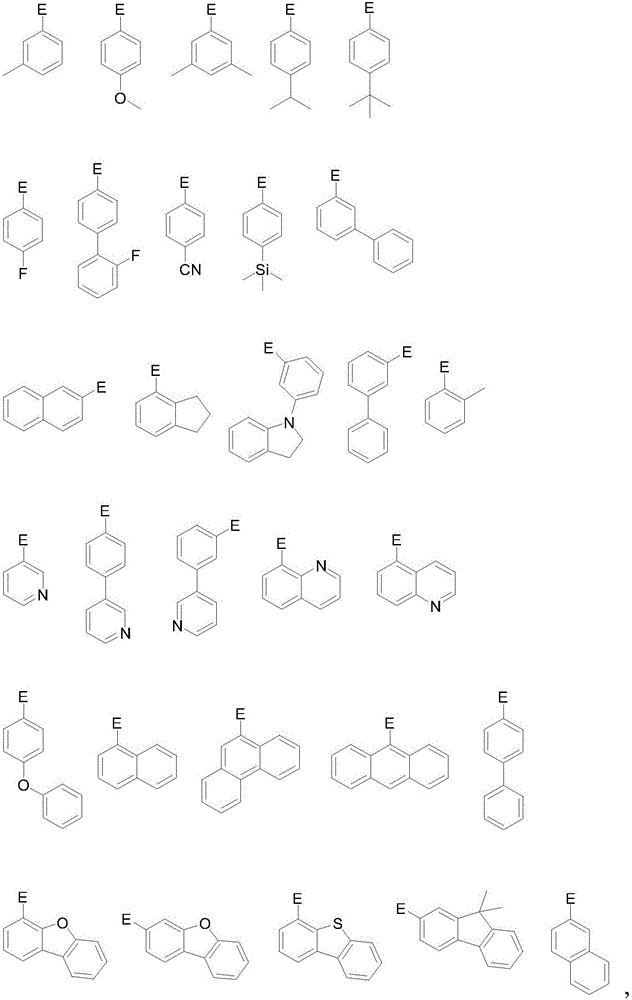
Organic electroluminescent material, preparation method and application thereof
An electroluminescent material and luminescent technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of slow progress in the research and development of blue light materials, achieve large-scale promotion, reduce fluorescence quenching, Highly stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] Preparation of intermediate a
[0039]
[0040] Under nitrogen protection, the raw materials 1,8-dibromonaphthalene (28.6g, 0.1mol), phenylboronic acid (12.2g, 0.1mol) and 180mL toluene, 75mL were added to a 500mL there-neck flask, and then the catalyst tetrakis (triphenyl) was added. phosphine) palladium (0.116 g, 0.1 mmol), acid binding agent potassium carbonate (20.7 g, 0.15 mol). The system was heated to reflux and stirred for 6 hours, then cooled to 20-25°C naturally, the solution was separated, the solvent was removed, and the crude product was crystallized with absolute ethanol to obtain 13.7 g of intermediate a with a yield of 48.4%.
[0041] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 16 H 11 Br, theoretical 282.0044, tested 282.0036. Elemental Analysis (C 16 H 11 Br), theoretical C: 67.87, H: 3.92, Br: 28.22, found C: 67.86, H: 3.93, Br: 28.22.
[0042] Preparation of intermediate b1
[0043]
[0044] 1) U...
Embodiment 1
[0070] The preparation of embodiment 1 compound C01
[0071]
[0072] 1) Under nitrogen protection, in a 250mL there-necked flask, add intermediate a (1.56g, 5.5mmol) and 50mL tetrahydrofuran, place in a low temperature bath and be cooled to -78°C, drip n-butyllithium (0.352g, 5.5mmol) , -78 ℃ reaction for 2 hours. Intermediate b1 (2.25 g, 5 mmol) was dissolved in 40 mL of tetrahydrofuran and dropped into the above reaction system, and reacted at -78°C for 2 hours. The temperature was naturally raised to 0-5° C., 20 mL of dilute hydrochloric acid was added to quench the reaction, the solution was separated, and the solvent was removed to obtain 2.65 g of intermediate C01-a with a yield of 87.7%. CO1-a (2.65g, 4.4mmol) was added into a 100mL there-necked flask, and 40mL of acetic acid and 0.5mL of 36% (wt%) concentrated hydrochloric acid were added, and the reaction was carried out under reflux at 110°C for 3.5 hours. After cooling down to 20-25°C naturally, suction filtrat...
Embodiment 2
[0076] The preparation of embodiment 2 compound C04
[0077]
[0078] The synthesis method refers to the preparation method of CO1.
[0079] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 60 H 39 NO 2 , the theoretical value is 805.2981, and the test value is 805.2978. Elemental Analysis (C 60 H 39 NO 2 ), theoretical value C: 89.41, H: 4.88, N: 1.74, O: 3.97, observed value C: 89.42, H: 4.87, N: 1.73, O: 3.98.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com