Preparation method and applications of forsythoside glucuronic acid derivative
A technology of glucuronic acid and forsythiatin, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problem of forsythiatin molecular instability, easy to change molecular configuration, and easy to be oxidized and other problems, to achieve the effect of convenient source of raw materials, easy quality control, and improved selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] 1. Preparation of forsythiatin-organic base complex
[0065] Add forsythiatin (4g, 10.75mmol) into dry anhydrous dichloromethane (180ml) at a temperature of 10°C, stir to dissolve and add organic base N,N-diisopropylethylamine 11.26mL (64.5mmoL), stirred for 10 minutes, carried out the hydroxyl activation reaction, and prepared the forsythiatin-organic base complex solution for later use, and the molar ratio of forsythiatin to organic base was 1:6;
[0066] In the process of the hydroxyl activation reaction in the present invention, controlling the reaction temperature at 0-20°C is applicable to the present invention, and the embodiment of the present invention takes 10°C as an example for illustration. In the present invention, except for the molar ratio of forsythiatin and organic base being 1:6, other molar ratios of 1:5-8 are applicable to the present invention. The embodiment of the present invention is described by taking 1:6 as an example.
[0067] The hydroxyl...
Embodiment 2
[0086] Except that the organic base 1,8-diazabicycloundec-7-ene (9.63 mL, 64.5 mmol) was added in step 1; the glycosyl donor in step 2 was 2,3,4-tri-O- The mass of benzoyl α-D bromoglucopyranose methyl ester is 43.89g (75.25mmoL), and the molar ratio of glycosyl donor to forsythialin is 7:1; catalyst silver carbonate (124.5g, 0.4515mol), except that the molar ratio of the catalyst to the sugar group donor was 6:1, the rest were the same as in Example 1 to obtain a white solid (compound 2, 3g), with a total yield of 79.8%.
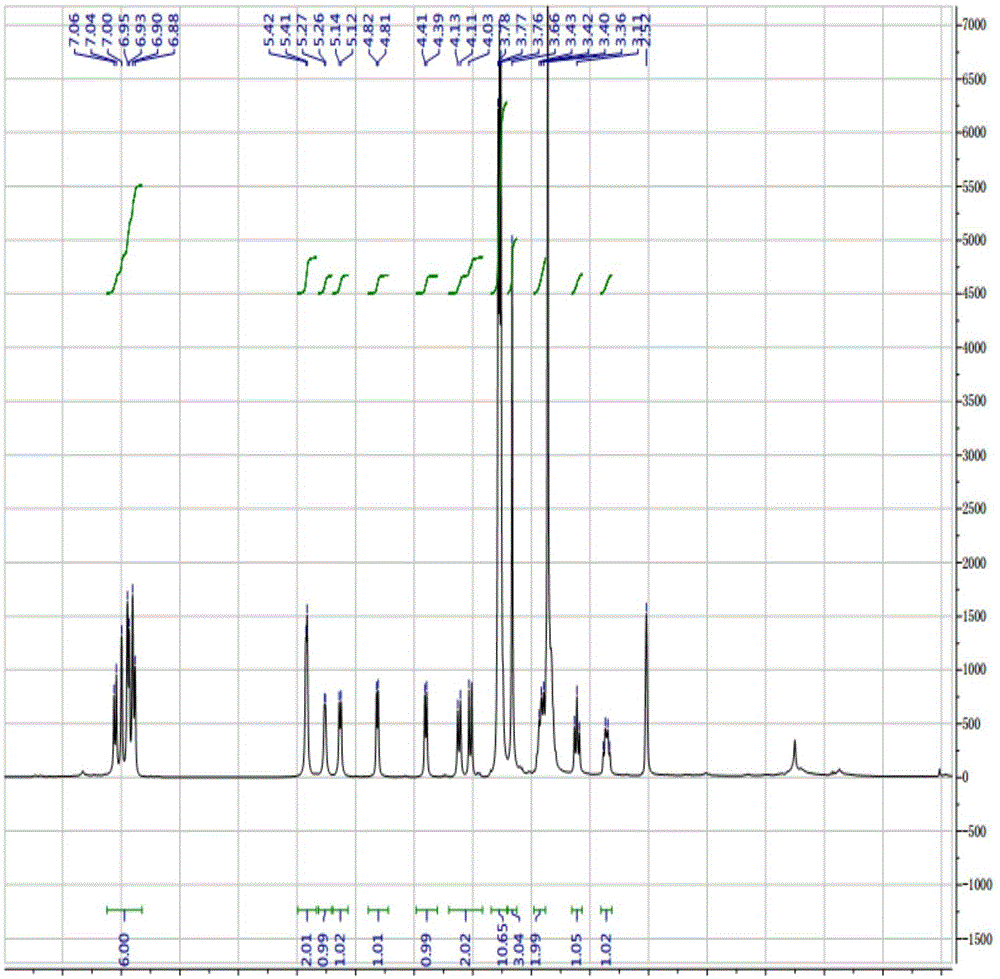
[0087] Compound 2 is a white solid, soluble in water and ethanol. Develop on a TLC plate (chromatographic solution is chloroform / methanol 3:1, Rf is 0.4) and then spray 10% H 2 SO 4 -Ethanol reagents are purple in color.
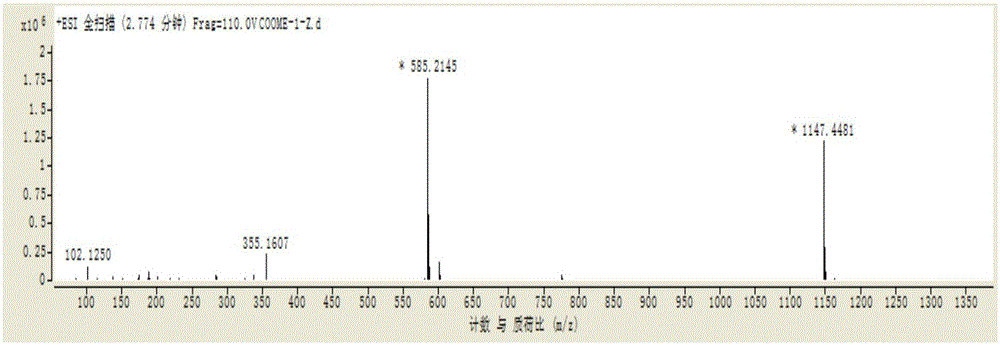
[0088] In the ESI-MS spectrum of compound 2, m / z[M-Na] - It is 547.18210 and the molecular weight is 570.52277.
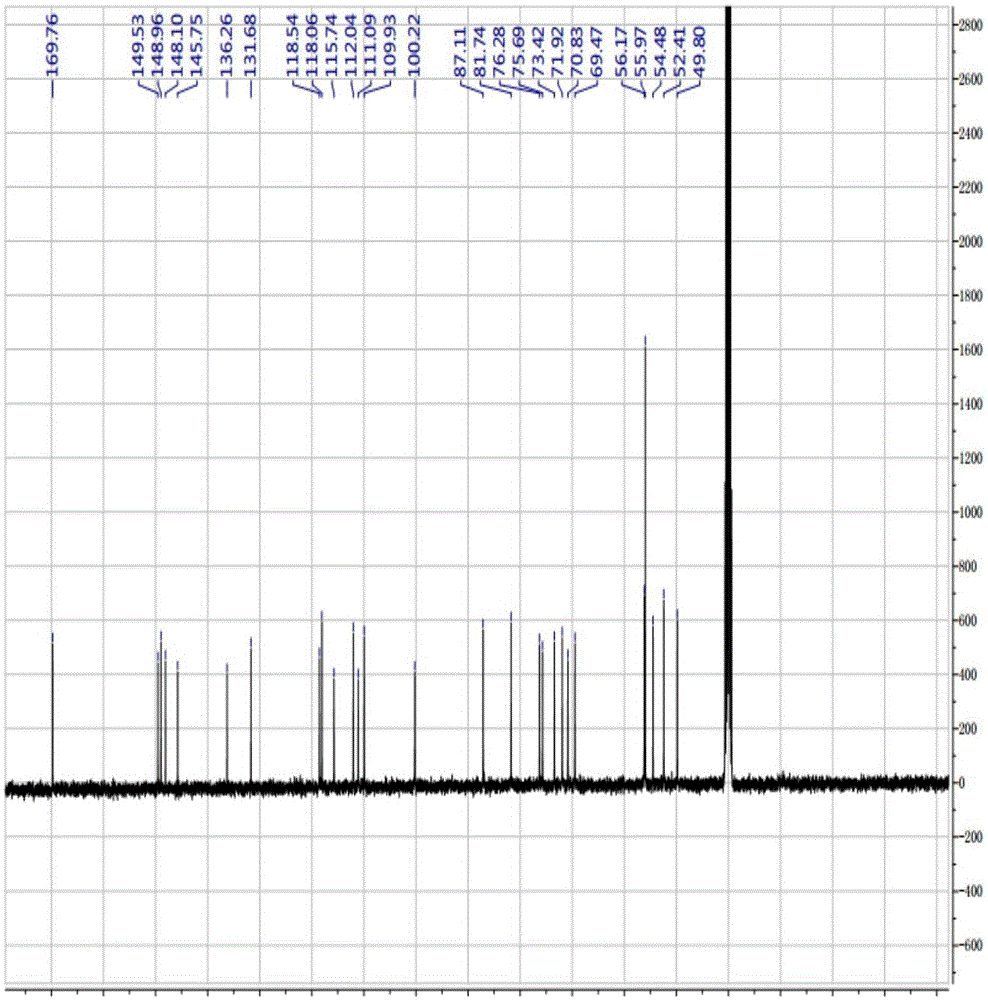
[0089] Compound 2 1 H-NMR, 13 C-NMR, IR are the same as compound 1 prepared in Example 1.
[0090] According to ESI-...
Embodiment 3
[0092] 1. Preparation of forsythiatin-organic base complex
[0093] Add forsythiatin (4g, 10.75mmol) into dry anhydrous dichloromethane (180ml) at a temperature of 0°C, stir to dissolve and add organic base 1,8-diazabicycloundec Carb-7-ene 9.63mL (64.5mmol), stirred for 20 minutes, carried out hydroxyl activation reaction, prepared forsythiatin-organic base complex solution for subsequent use;
[0094] 2. Glycosidation reaction
[0095] 2-1) 6.4g (16.125mmoL) of 2,3,4-tri-O-acetyl α-D bromoglucopyranose methyl ester and 17.78g (64.5 mmoL) was dissolved in dichloromethane, stirred uniformly to obtain glycosidation reagent;
[0096] 2-2) Add the forsythiatin-organic base complex solution into the glycosylation reagent while stirring at 0°C and nitrogen atmosphere, then heat up to 20°C, and keep the temperature at 20°C , carry out glycosylation reaction 10h, make glycosylation reaction mixture;
[0097] 3. Deacylation reaction
[0098] Under stirring, the glycosidation react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com