A kind of sericin conductive hydrogel and preparation method thereof and stent prepared therefrom
A technology of conductive hydrogel and sericin, which is applied in pharmaceutical formulations, prostheses, drug delivery, etc., to achieve the effects of reducing trauma and pain, excellent electrical conductivity, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
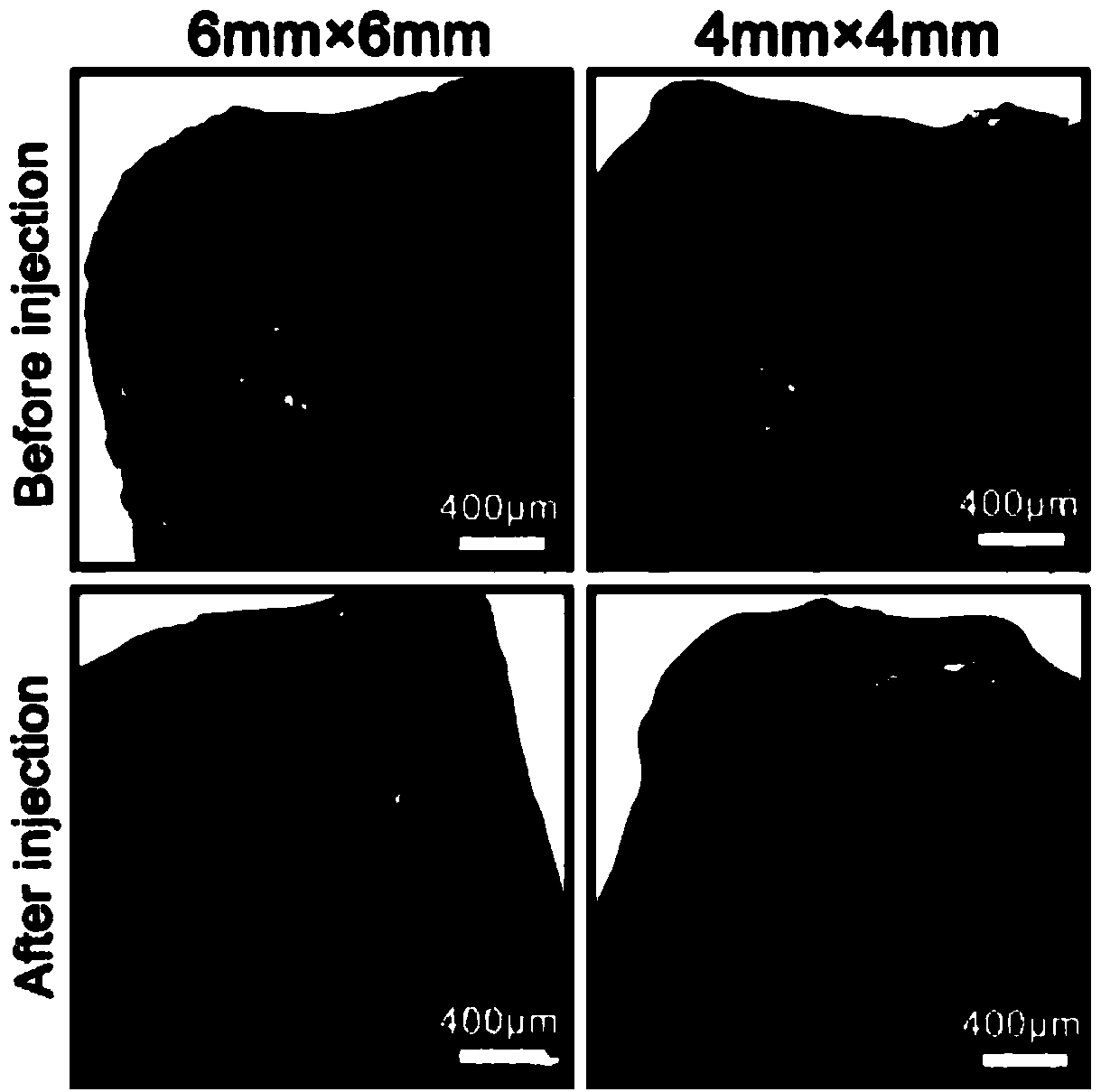
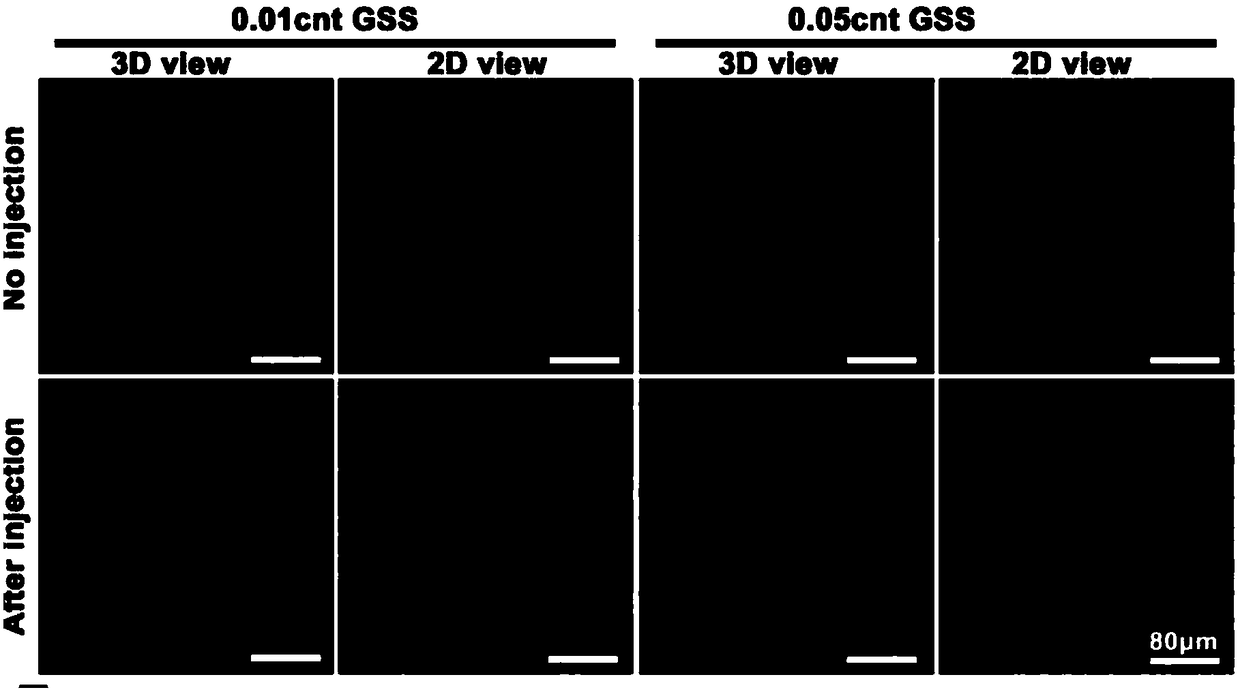
Examples
Embodiment 1-4
[0047] The preparation of embodiment 1-4 sericin conductive hydrogel
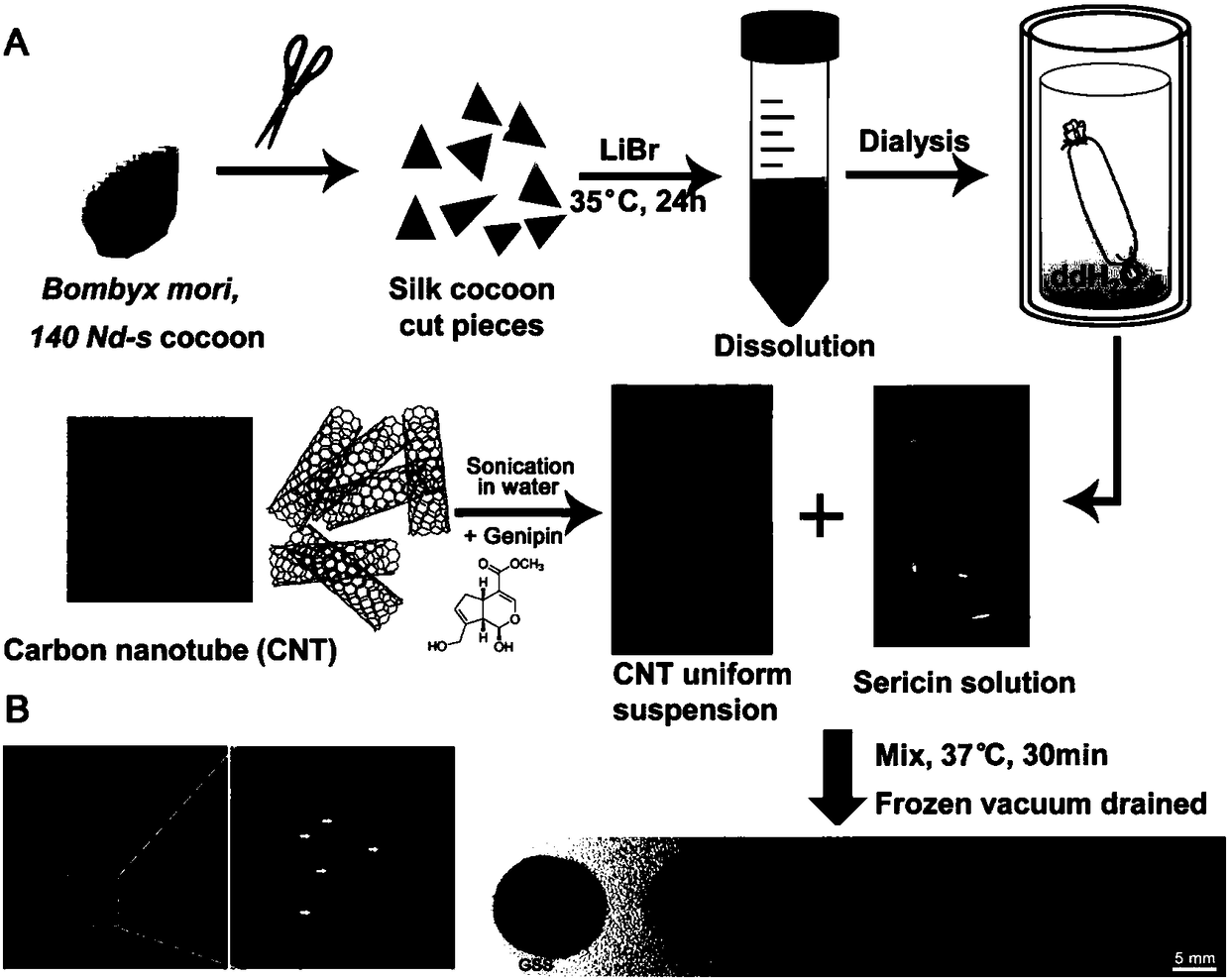
[0048] The preparation process of the sericin conductive hydrogel of the present embodiment is as follows: figure 1 shown.
[0049] 1. Preparation of sericin aqueous solution
[0050] The silk used in this example comes from the silkworm cocoon of the silkworm silk fibroin-deficient mutant (purchased from the Institute of Sericulture, Chinese Academy of Agricultural Sciences). An aqueous solution of sericin was prepared from the cocoons by the following method:
[0051] 1) Weigh 1 g of silkworm cocoon of mutant silkworm and cut it into 1 cm 2 The fragments were placed in a clean beaker, washed 3 times with ultrapure water, and centrifuged at 3500rpm for 5 minutes to remove water;
[0052] 2) Add 55 mL of LiBr aqueous solution with a concentration of 6 mol / L to the silkworm cocoon fragments obtained in step 1), put the beaker into a constant temperature water bath at 35° C. for 24 hours to dissolve sericin...
Embodiment 9-12
[0121] The preparation of embodiment 9-12 horseradish peroxidase (HRP) sustained-release agent
[0122] During the preparation of Example 1-4, the HRP solution was added so that the content of HRP in the final scaffold was 8 μg; the rest of the operations were the same as the preparation of the hydrogel in Example 1-4, thus obtaining four kinds of hydrogels containing HRP. These four sericin conductive hydrogels containing HRP were freeze-dried by the scaffold preparation method in Examples 5-8 to form the HRP slow-release agents of Examples 9-12.
[0123] Put the above slow-release agent in 1 mL of PBS with pH 7.4 at 37°C; , 264, 288, and 323 hours, take out the supernatant carefully, and add 1mL PBS (pH7.4) again; use enzyme-linked immunosorbent assay (ELISA) to measure the content of HRP in the supernatant, and calculate the accumulated HRP in the supernatant at each time point The ratio of the HRP content and the actual drug load is the cumulative release rate, the result...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com