Ring-shaped RNA circ-NFATC3 and application thereof
A circular and versatile technology, applied in the fields of molecular biology and oncology, can solve the problems of difficult early diagnosis, difficult early diagnosis of liver cancer, and low surgical cure rate, and achieve accurate diagnosis of liver cancer, reduced cell migration rate and cell invasion ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
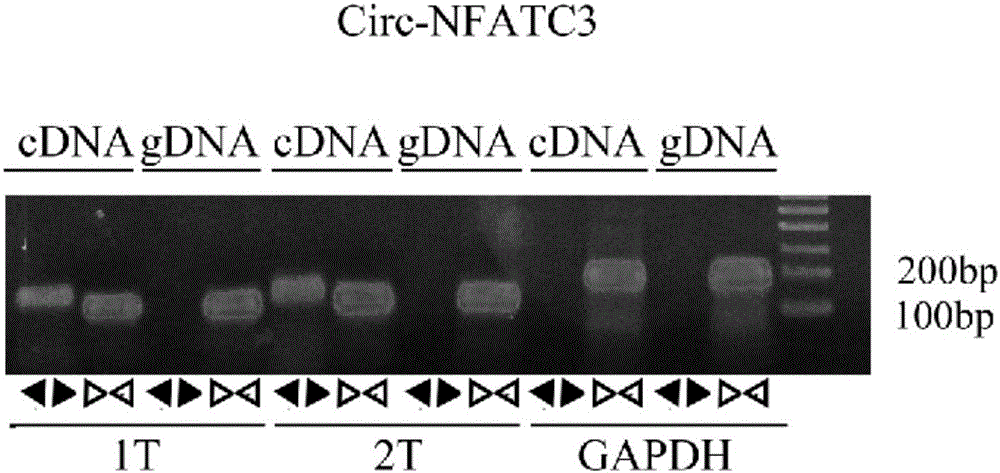
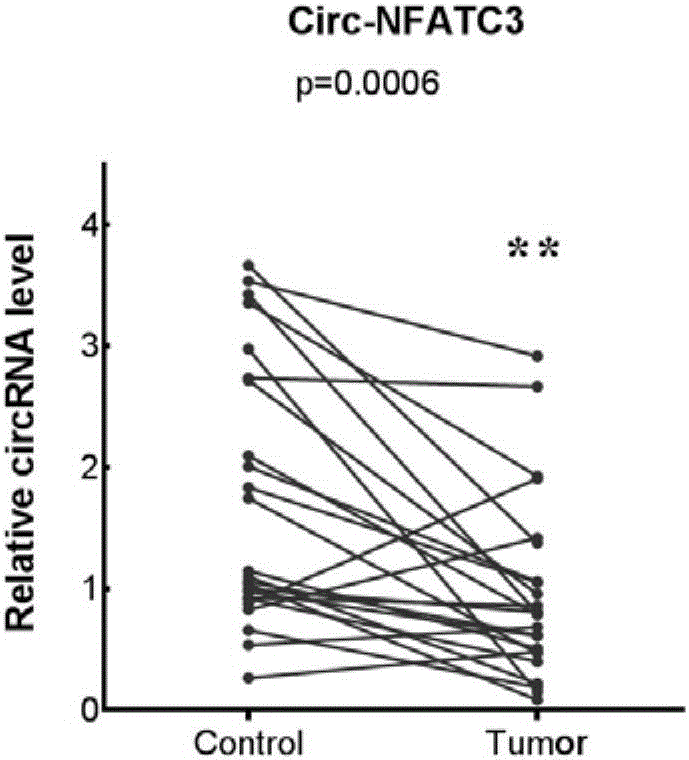
[0030] Example 1: RT-PCR reaction detection of circ-NFATC3 gene expression in liver cancer tissue.
[0031] The specific experimental plan is as follows:
[0032] 1. RNA extraction
[0033] 1) Tissue processing: Take about 10 mg of tissue and add 1 ml Trizol, homogenize with a homogenizer; centrifuge for 15 minutes at 12000 g, and take the supernatant.
[0034] 2) Add 200ul chloroform to the supernatant, mix vigorously up and down for half a minute, and let stand for 3 minutes.
[0035] 3) Centrifuge at 12,000g for 15 minutes at 4°C. At this time, it can be seen that the lysate is divided into three layers: the upper layer is RNA in the aqueous phase; the middle layer is DNA, lipids, etc.; the lower layer is cell residues, proteins, polysaccharides, etc.
[0036] 4) Take the supernatant into a new EP tube; add an equal volume of isopropanol, mix well, let stand for 10 minutes, and then centrifuge at 12000g for 10 minutes at 4°C.
[0037] 5) Carefully remove the supernatant,...
Embodiment 2
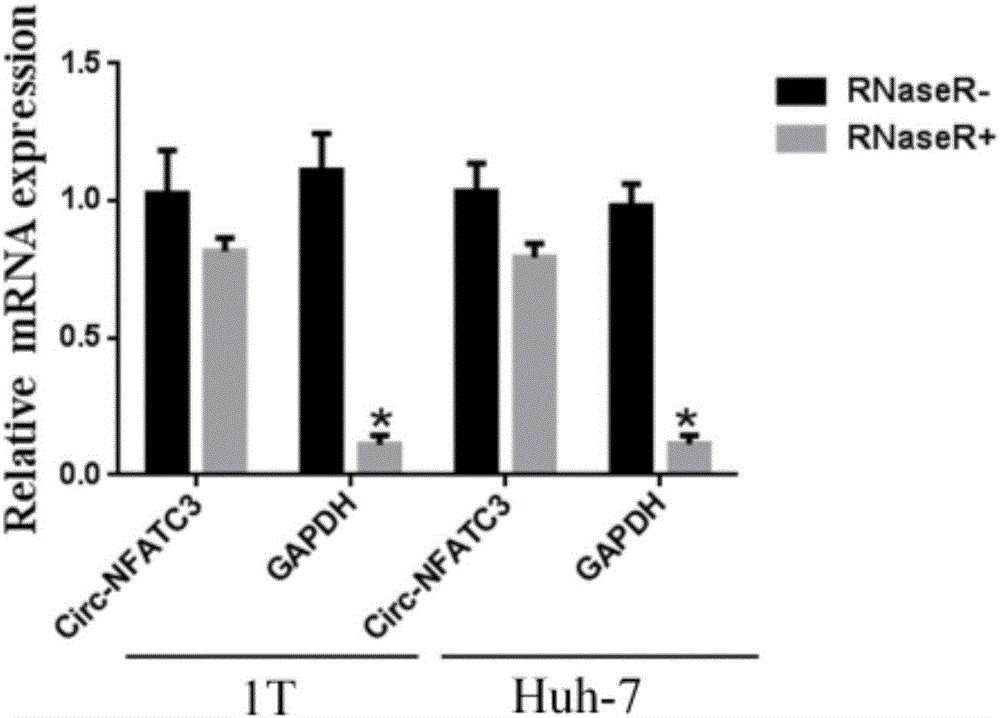
[0048] Example 2: Detecting the expression of circ-NFATC3 in liver cancer by QPCR
[0049] 1. RNA extraction: with embodiment 1;
[0050] 2. cDNA reverse transcription: same as Example 1;
[0051] 3. QPCR amplification experiment
[0052] 1) Experimental system:
[0053]
[0054] The circ-NFATC3 divergent primer in Example 1 was used to amplify circular RNA circ-NFATC3, and the hsaGAPDH convergent primer in Example 1 was used to amplify the internal reference gene.
[0055] 2) Reaction conditions:
[0056] Step 1: 95°C for 2 minutes
[0057] Step 2 (40 cycles): 95°C for 3 seconds, 60°C for 30 seconds
[0058] The third step 60-95 ℃ melting curve
[0059] 3) Amplify the target gene on the machine
[0060] 4) qPCR relative quantitative results
[0061] The formula for calculating the relative expression of the target gene is: 2-△△Ct=2-[(△Ct)Test-(△Ct)Control]. Ct object is the Ct value of the target gene, and Ct housekeeper is the Ct value of the housekeeping gene. ...
Embodiment 3
[0064] Example 3: Overexpression of circ-NFATC3 lentivirus and its stable cell line construction
[0065] 1. Construction of overexpressed circ-NFATC3 lentiviral vector: The complete linear sequence of circ-NFATC3 was synthesized in Shanghai Jierui Company, and the sequence was annealed into a double-stranded DNA fragment, which was inserted into the LVminiCirc vector through multiple cloning sites, and the recombinant plasmid was identified by sequencing , Control Negative control is LVminiCirc empty vector without insert sequence.
[0066] 2. Lentiviral packaging
[0067] (1) 24 hours before transfection, digest 293T cells in the logarithmic growth phase with trypsin, transfer to 10cm cell culture dish, 37°C, 5% CO 2 Cultured in an incubator. After 24 hours, when the cell density reaches 70%-80%, it can be used for transfection. Cell state is critical for virus packaging, so good cell state and low passage times need to be guaranteed.
[0068] (2) Replace the cell cultur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com