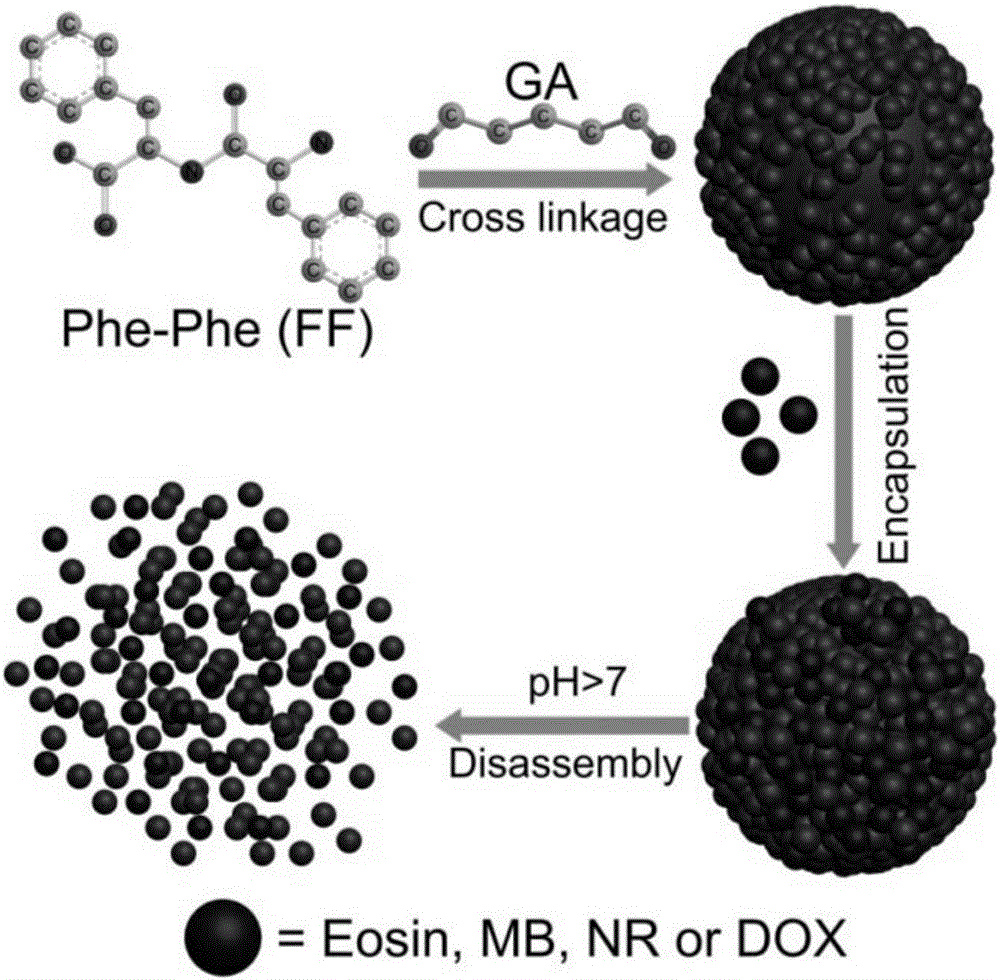
Nano carrier capable of pH-responsive rapid release of object molecules, and preparation method and application thereof
A nano-carrier and guest molecule technology, applied in the field of nano-materials, to achieve the effect of easy operation, good stability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, the preparation of diphenylalanine nanocarrier
[0052] 1) Dissolve 1 mg of biological peptide diphenylalanine in 8 μl of hexafluoroisopropanol to obtain a hexafluoroisopropanol solution of biological peptide (also called solution a), with a concentration of 125 mg / ml, and store at 4°C for 24 hours above to make it fully dissolved;
[0053] 2) Dilute 2.4 μl of an aqueous solution of 25% glutaraldehyde by ultrapure water to 500 μl to obtain solution b with a concentration of 0.12%; the molar ratio of diphenylalanine to glutaraldehyde is 1 :1;
[0054] 3) Heat solution b in a water bath at 65°C until fully dissolved at a constant temperature, then add it to solution a, react with Schiff base, leave at room temperature (25°C) for 24 hours after aging, the solution turns into light yellow turbid liquid, and remove by centrifugation The supernatant liquid is the pH-responsive nanocarrier capable of rapidly releasing guest molecules of the present invention. ...
Embodiment 2
[0055] Embodiment 2, preparation of phenylalanine nanocarrier
[0056] 1) Dissolve 20 mg of biological amino acid phenylalanine in 1 mL of ultrapure water to obtain an aqueous solution of phenylalanine (also called solution a) with a concentration of 20 mg / ml, store at 4°C for more than 24 hours to fully dissolve;
[0057] 2) Take 24 μl of an aqueous solution of glutaraldehyde with a concentration of 25% by mass, i.e. solution b, with a concentration of 0.6%; the molar ratio of phenylalanine to glutaraldehyde is 1:1;
[0058] 3) Add solution b to solution a, react with Schiff's base, and after aging at room temperature (25°C for 24 hours, the solution turns into a light yellow turbid liquid, and centrifuge to remove the supernatant liquid to obtain the pH-responsive pH-responsive pH solution of the present invention. Nanocarriers for Rapid Release of Guest Molecules.
Embodiment 3
[0059] Embodiment 3, the characterization of the diphenylalanine nano-carrier obtained in Example 1 of the present invention
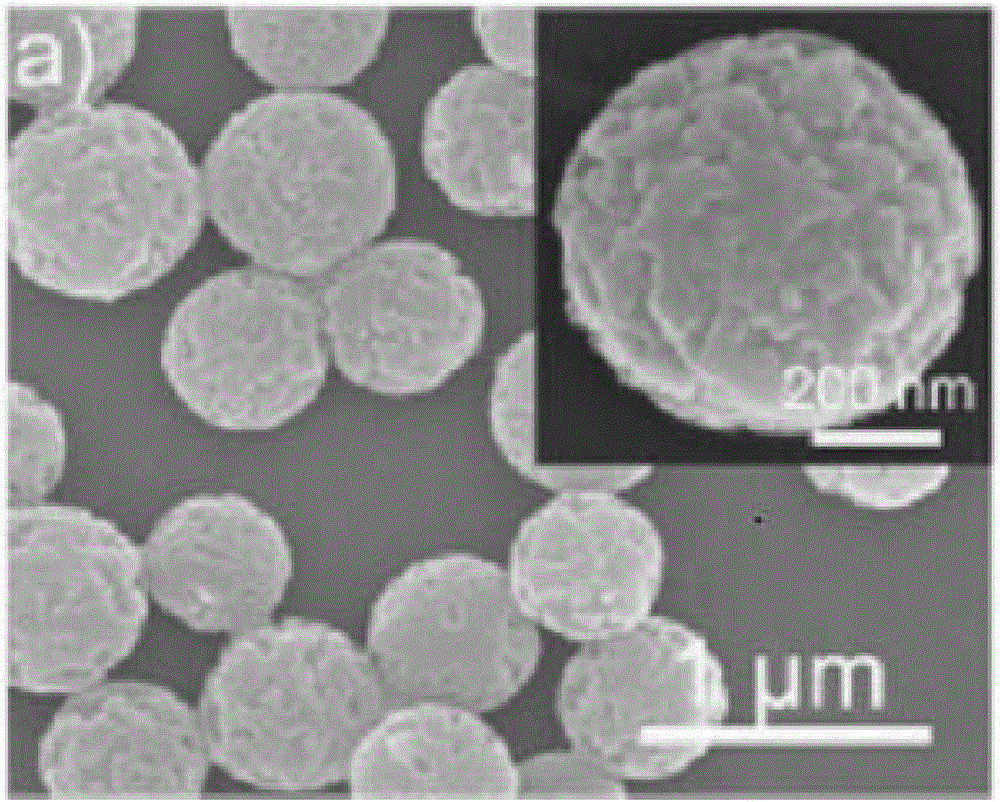
[0060] Scanning electron microscope characterization of the nano-carrier prepared in Example 1 of the present invention: drop the nano-container suspension (5 μl) that has been centrifuged and washed on the surface of the silicon wafer, and after vacuuming it to dryness, fix it on the sample stage with conductive glue, and After spraying Au particles on its surface with a radiometer, observe it under a HITACHI S-4800 scanning electron microscope, and the accelerating voltage is 10kV; the results are as follows figure 2 shown.
[0061] Depend on figure 2 It can be seen that the nano-carrier material obtained in the present invention is a monodisperse nano-sphere, the surface is very rough and has many pore structures.
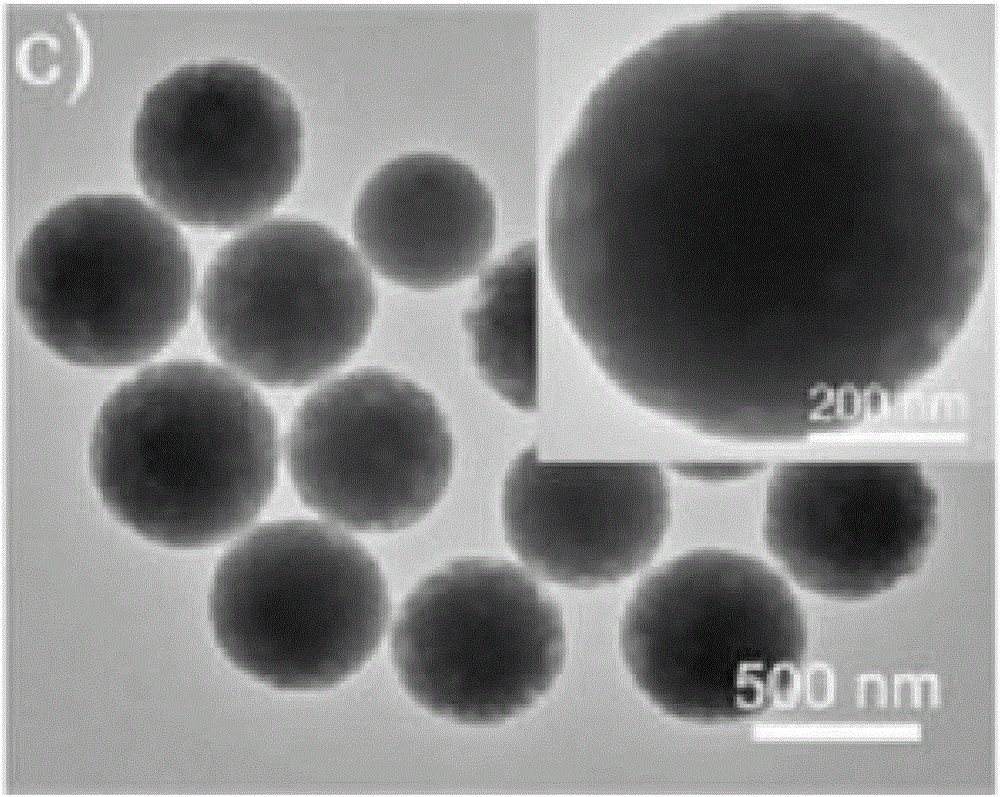
[0062] Transmission electron microscopy characterization of diphenylalanine nanocarriers: drop the centrifuged-washed nanosphere su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com