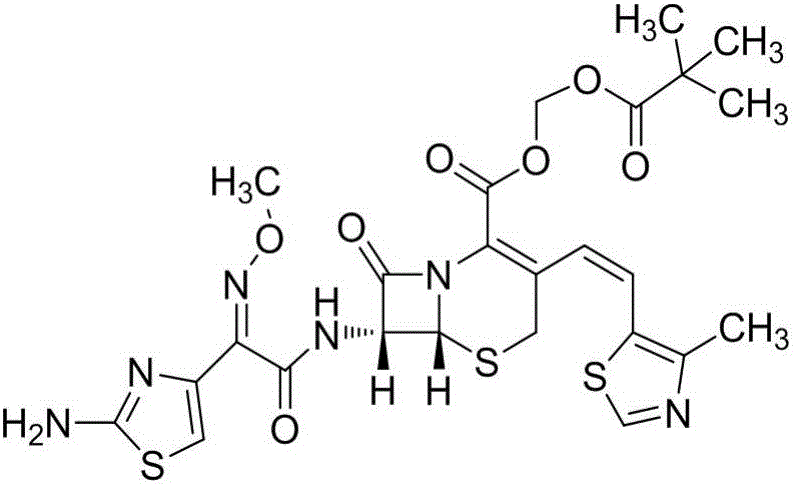
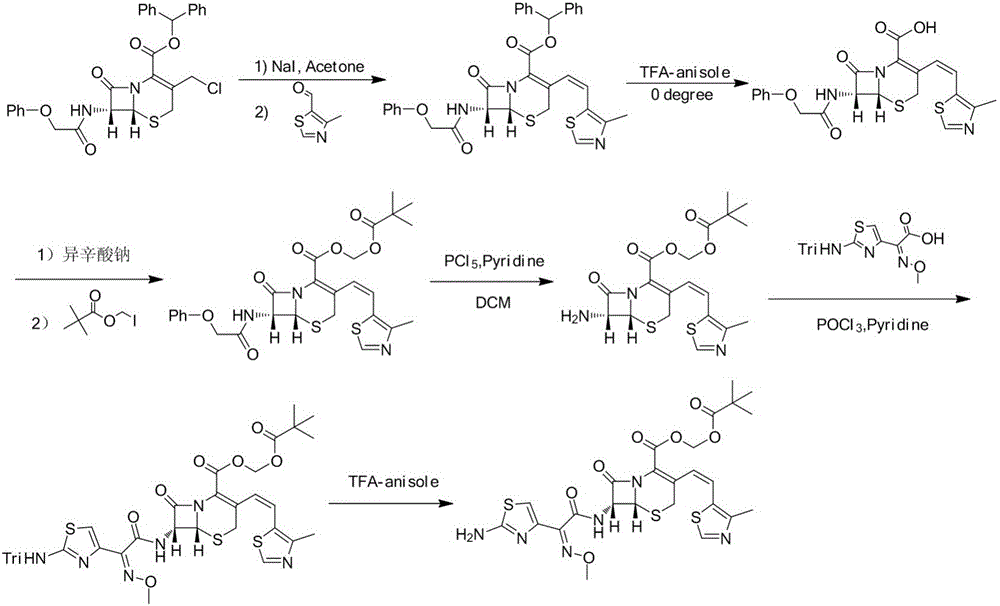
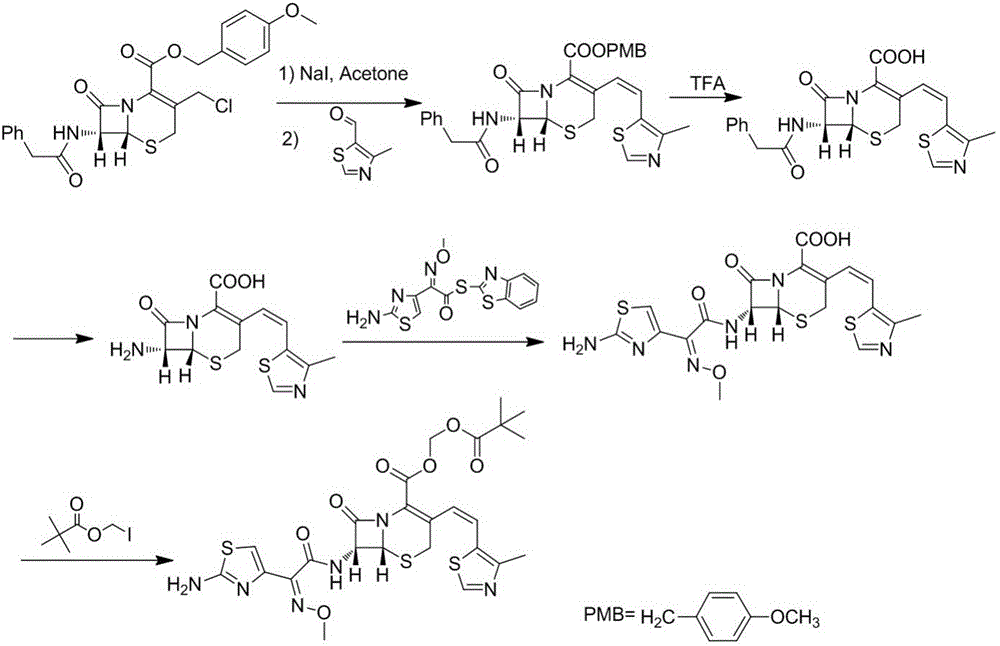
Cefditoren pivoxil purifying method
A technology of cefditoren pivoxil and a refining method, which is applied in the field of medicine and chemical industry, can solve the problems of low product purity and cumbersome industrial production, and achieve the effect of simple operation and easy industrial production operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of cefditoren pivoxil refined product
[0032] (1) Add 70mL ethyl acetate and 14g crude cefditoren pivoxil to a 250mL reaction flask, stir and cool to 0°C, add 98mL 5% hydrochloric acid solution, stir for 10min, and separate the liquids; The solution was extracted once, the water layers were combined, the water layer was washed with 70mL ethyl acetate, and the layers were separated, and the obtained water layer was cefditoren pivoxil salt solution; 70mL dichloromethane was added to the water layer, stirred and cooled to 0°C, Add 8% sodium bicarbonate solution, adjust the pH to 5.0 within 1 h, separate the layers, wash the organic layer twice with saturated aqueous sodium chloride solution, 100 mL each time, add 4.2 g of anhydrous magnesium sulfate and 1.4 g of drug Use activated carbon, dry and decolorize at 0°C for 0.5h, filter, wash the filter cake with 10mL of dichloromethane, combine the filtrate and washing liquid, the filtrate is an o...
Embodiment 2
[0035] Embodiment 2: the preparation of cefditoren pivoxil refined product
[0036](1) Add 100mL n-butyl acetate and 20g crude cefditoren pivoxil to a 500mL reaction flask, stir and cool down to 25°C, add 120mL 5% sulfuric acid solution, stir for 10min, and separate the liquids; Extract with sulfuric acid solution once, combine the water layers, wash the water layer with 100mL n-butyl acetate, separate the layers, the obtained water layer is cefditoren pivoxil salt solution; add 100mL1,2-dichloroethane to the water layer, stir Cool down to 5°C, add 4% sodium hydroxide solution dropwise, adjust the pH to 8.0 within 1 h, separate the layers, wash the organic layer twice with saturated aqueous sodium chloride solution, 150 mL each time, add 5.0 g of anhydrous Magnesium sulfate and 2.0g of pharmaceutical activated carbon, dry and decolorize at 10°C for 0.5h, filter, wash the filter cake with 15mL of 1,2-dichloroethane, combine the filtrate and wash, the filtrate is the organic sol...
Embodiment 3
[0039] Embodiment 3: the preparation of cefditoren pivoxil refined product
[0040] (1) Add 70mL of dichloromethane and 14g of crude cefditoren pivoxil to a 250mL reaction flask, stir and cool down to -10°C, add 98mL of 5% nitric acid solution, stir for 10min, and separate the liquids; Nitric acid solution was extracted once, and the aqueous layers were combined; the aqueous layer was washed with 70 mL of dichloromethane, and separated, and the obtained aqueous layer was cefditoren pivoxil salt solution; 70 mL of ethyl acetate was added to the aqueous layer, stirred and cooled to 5 ° C, Add 8% sodium carbonate solution dropwise, adjust the pH to 7.0 within 1 h, separate the liquids, wash the organic layer twice with saturated aqueous sodium chloride solution, 100 mL each time, add 4.2 g of anhydrous magnesium sulfate and 1.4 g of medicine to the organic layer. Use activated carbon, dry and decolorize at 0°C for 0.5h, filter, wash the filter cake with 10mL ethyl acetate, combin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com