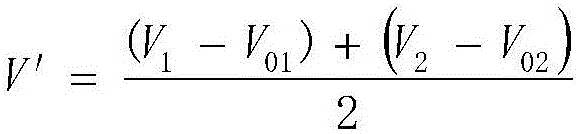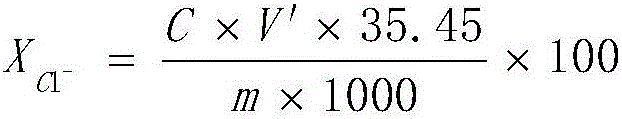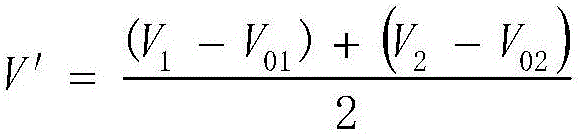Method for measuring chloride ions in polycarboxylate superplasticizer
A measurement method and technology of water reducer, applied in chemical method analysis, chemical analysis by titration method, measuring device, etc., can solve the problems of ineffective separation of chloride ion and thiocyanate, error of test results, etc., and achieve large-scale equipment Cost advantage, high precision, and the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Take the batch numbers J160412-1, J160610-1, J160713-1, and J160718-1 of the batch numbers of the theoretical values of the formulas produced by our company respectively 0.013%, 0.015%, 0.020%, and 0.015%, and test them according to the following steps Chloride content.
[0047] First, determine the endpoint potential E corresponding to the titration endpoint ep
[0048] Weigh 0.1000g of sodium chloride, put it in a 400mL beaker, add 4mL of nitric acid solution (1+1), add water to 200mL, add 10mL of starch solution with a concentration of 10g / L, put in a magnetic stirrer, and place the beaker under a magnetic force On the stirrer, insert the silver electrode and reference electrode connected to the potentiometric titrator, turn on the magnetic stirrer, then add dropwise the silver nitrate standard solution with a concentration of 0.1000mol / L, record the burette reading V and the corresponding silver electrode potential E ;
[0049] When the titration is close to th...
Embodiment 2
[0080] For the four batches of water reducer samples whose batch numbers are J160412-1, J160610-1, J160713-1, and J160718-1 in Example 1 of the present invention, the chloride ion content was measured by the method of GB / T 8077-2012 respectively, and the obtained Results of Table 2:
[0081] Table 2:
[0082] Sample lot number
[0083] As can be seen from Table 2, the chloride ion content measured by the method of the present invention is closer to the theoretical value, and therefore has higher accuracy than the method of GB / T8077-2012.
Embodiment 3
[0085] Take the water reducer sample with the batch number J160713-1, weigh 40.0000g in six parts each, add a certain amount of chloride ion standard sample, and use the improved method to carry out the recovery rate test. The test results are shown in Table 3:
[0086] table 3:
[0087] serial number
The amount of chloride ion added, mg
Measured value, mg
Recovery amount, mg
Recovery rate,%
1
40.0000
0.00
7.2
/
/
2
40.0000
5.00
12.1
4.9
98.00
3
40.0000
10.00
17.3
10.1
101.00
4
40.0000
15.00
22.1
14.9
99.33
5
40.0000
20.00
27.3
20.1
100.50
6
40.0000
25.00
32.2
25.0
100.00
[0088] Wherein, the preparation of chloride ion standard sample is as follows: Accurately weigh 1.6485g of sodium chloride burned to constant weight in a high-temperature furnace at 500°C to 600°C, dissolve it in a 1000ml volumetric f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com