Preparation method of high-purity delafloxacin meglumine salt
A delafloxacin, high-purity technology, applied in the field of medicinal chemistry, can solve the problems of solvent residue, low yield, unsuitable for industrial production, etc., and achieve the effect of simple operation, high yield and remarkable refining effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
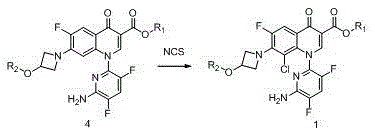
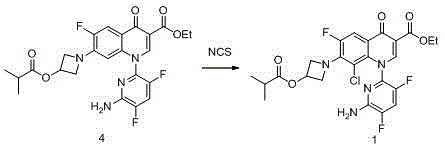
[0043] Embodiment 1 The preparation of the compound of formula 1, wherein, R1 is ethyl, R2 is isobutyryl, and the reaction formula is as follows:
[0044]
[0045]In a 100L reactor, add 5.0Kg (9.9mol) of the compound of formula 4, 25Kg ethyl acetate and 500g sulfuric acid in sequence, stir at room temperature, and slowly add dropwise a solution of 1.7Kg NCS (12.8mol) dissolved in 25Kg methyl acetate. After the dropwise addition, the mixture was stirred at room temperature for 5 hours, washed with sodium bicarbonate solution, sodium sulfite solution and saturated brine, and the organic phase was concentrated to dryness to obtain 5.0 Kg of the crude compound of formula 1 with a purity of 97%.
Embodiment 2
[0046] Embodiment 2 The purification of the compound of formula 1
[0047] Put 1Kg of the crude compound of formula 1 prepared in Example 1 into a 20L reaction flask, add 2L methanol, 3L water, heat to 50°C, turn off the heating after stirring for 1 hour, slowly cool down to room temperature, crystallize in an ice-water bath for 5h, pump After filtering, the filter cake was washed with a small amount of cold methanol, sucked dry, and the filter cake was dried under reduced pressure to constant weight to obtain 930 g of a light yellow powder with a yield of 93% and an HPLC purity of 99.1%.
Embodiment 3
[0048] Embodiment 3 The refining of formula 1 compound
[0049] Put 1Kg of the crude compound of formula 1 prepared in Example 1 into a 20L reaction flask, add 6L ethanol and 2L water, heat to 60°C and stir for 1 hour, then turn off the heating, slowly cool down to room temperature, crystallize in an ice-water bath for 7h, and filter with suction. The filter cake was washed with a small amount of cold ethanol, sucked dry, and the filter cake was dried under reduced pressure to constant weight to obtain 900 g of a light yellow powder with a yield of 90% and an HPLC purity of 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com