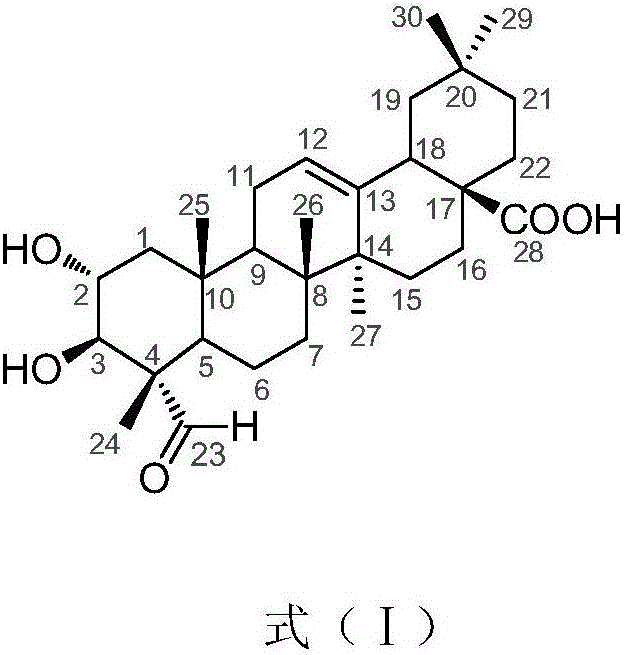
Application of compound 2alpha,3beta-dyhydroxy-23-formyl-olive-12-ene-28-acid in preparation of glycosidase inhibitor medicine
A technology of glycosidase inhibitor and compound, which is applied in the field of natural medicinal chemistry, can solve the problems such as the glycosidase inhibitor activity that has not been seen, and achieves the effect of easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
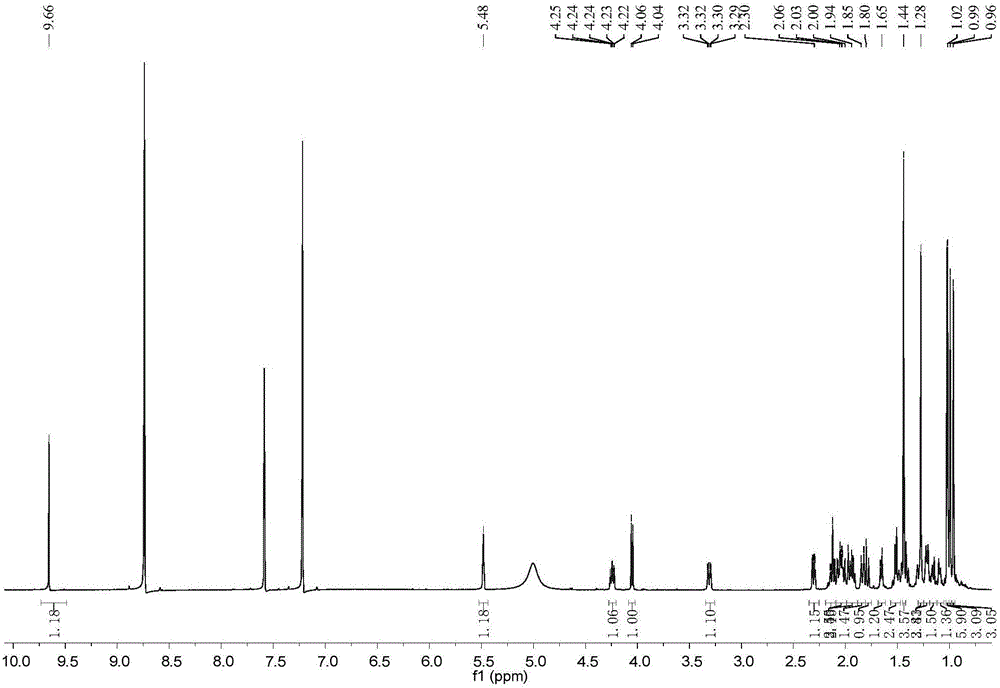
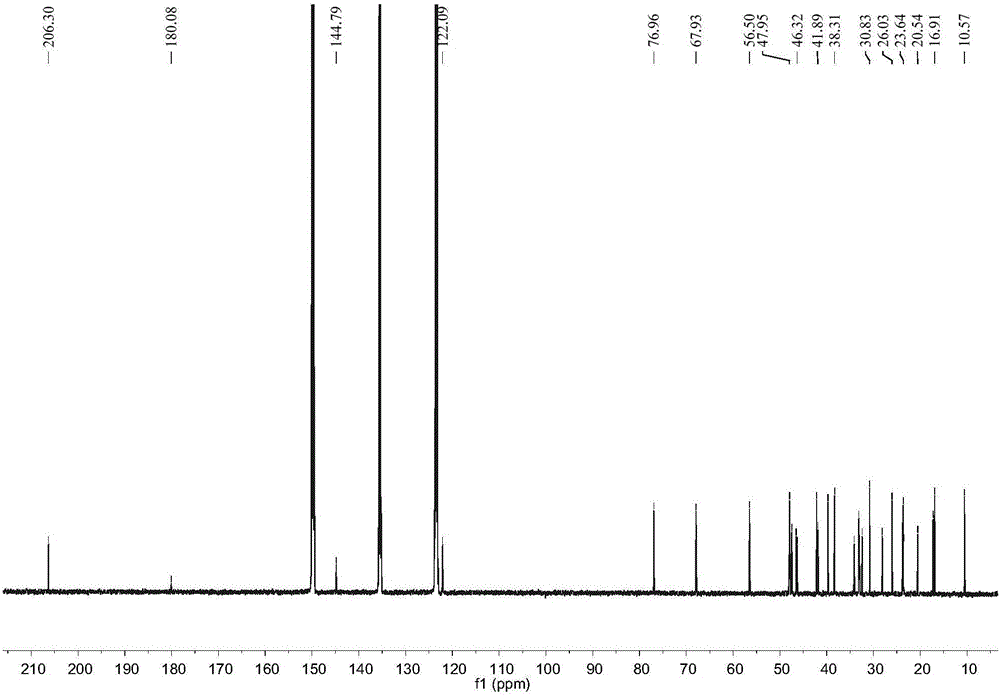
[0020] Example 1: Preparation of compound 2α, 3β-dihydroxy-23-formyl-olean-12-ene-28-acid
[0021] 1.1 Extraction and separation
[0022] The sample (dried fruit of Akebia trifoliata, weighing 2.0 kg) was pulverized and extracted three times with 95% ethanol aqueous solution at room temperature, and the combined filtrate was concentrated under reduced pressure to remove the organic solvent to obtain the crude extract of the total extract. The total extract crude extract was suspended in 500ml water, then extracted with an equal volume of petroleum ether and then extracted 3 times with ethyl acetate, and the ethyl acetate extract was concentrated under reduced pressure to obtain the total extract of ethyl acetate (170g). Dissolve the total extract of ethyl acetate with chloroform / methanol (350mL) with a volume ratio of 1:1, add normal phase silica gel (80-100 mesh) and mix the sample with a weight ratio of 1:1.5, evaporate to dryness, and dry-pack the column (200 -300 mesh, 16...
Embodiment 2
[0025] Example 2: Compound 2α, 3β-dihydroxy-23-aldehyde-olean-12-ene-28-acid inhibits α-glucosidase activity detection
[0026] 2.1 Instruments and reagents
[0027] The experiment used Genois microplate reader (Tecan GENios, Swizerland); α-glucosidase was purchased from Sigma Chemical Co. (Sigma-Aldrich, St.Louis, USA), and acarbose (Acarbose) was purchased from Tokyo Chemical Industry Co., Ltd. (Japan), 4-nitrophenol-α-D-glucopyranoside (PNPG) was purchased from Tokyo Chemical Industry Co., Ltd. (Japan); sample 2α, 3β-dihydroxy-23- Aldehyde-olean-12-en-28-acid was prepared from the above experimental example.
[0028] 2.2 Test method:
[0029]a) Preparation of drug solution: prepare 10 mg / ml of 2α, 3β-dihydroxy-23-aldehyde-olean-12-en-28-acid and acarbose from dimethyl sulfoxide (DMSO) respectively solution, and prepare 67mmol / L of phosphate buffer (prepared with ultrapure water), PNPG substrate solution (5mM, prepared with phosphate buffer), and 0.2M NaCO 3 solution (pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com