A method for recovering cryolite, calcium carbonate and sodium sulfate from calcium fluoride sludge resources
A technology of sodium sulfate and calcium fluoride, applied in the preparation of calcium carbonate/strontium/barium, aluminum fluoride, sulfate/bisulfate, etc., can solve the problems of complex process conditions and high toxicity of hydrogen fluoride gas, and achieve easy scale The effect of chemical production, high conversion rate recovery, and easy control of process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
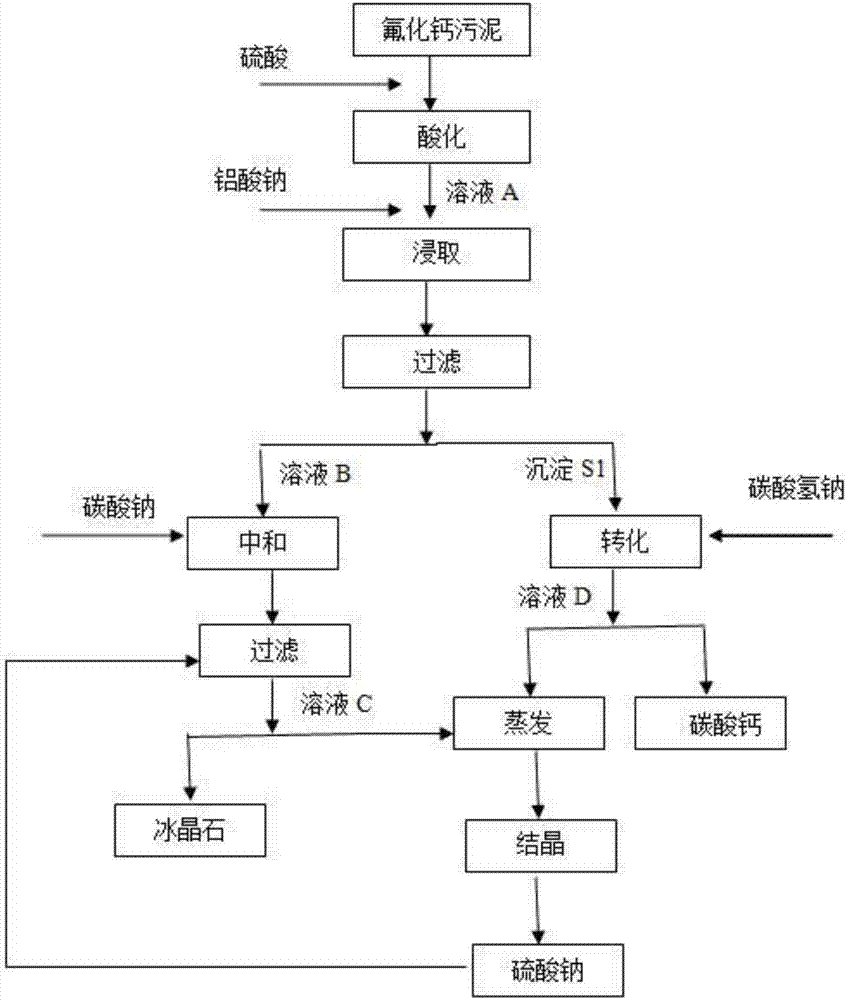
[0027] The applicant provides a method for reclaiming cryolite, calcium carbonate and sodium sulfate from calcium fluoride sludge resources, the specific steps are as follows:
[0028] (1) add sulfuric acid in calcium fluoride sludge 1000g, adjust its pH=2, when using sulfuric acid to acidify sludge, the time is 1.5h; Calcium that exists in the form of calcium hydroxide and calcium carbonate is converted into calcium sulfate to obtain solution A.
[0029] (2) Add 200g of sodium aluminate solid in solution A, and carry out stirring and leaching to convert calcium fluoride into fluoaluminic acid and calcium sulfate precipitation S1 and solution B;
[0030] (3) Sodium carbonate is added in solution B to make it pH=0.1, and 70g of sodium sulfate is added to promote the precipitation of sodium fluoroaluminate completely, and the stirring time is 1h to obtain 100g of cryolite and solution C; the amount of sodium carbonate is 1.3 of calcium sulfate times.
[0031] (4) Add 100mL 20w...
Embodiment 2
[0035] The applicant provides a method for reclaiming cryolite, calcium carbonate and sodium sulfate from calcium fluoride sludge resources, the specific steps are as follows:
[0036] (1) Add sulfuric acid to 1000 g of calcium fluoride sludge to adjust its pH=1. When sulfuric acid is used to acidify the sludge, the time is 1 hour; the calcium existing in the form of calcium hydroxide and calcium carbonate in the sludge is converted into sulfuric acid Calcium, to obtain solution A.
[0037] (2) Add 285g of sodium aluminate solid in solution A, and carry out stirring and leaching to convert calcium fluoride into fluoaluminic acid and calcium sulfate precipitation S1 and solution B;
[0038] (3) Sodium carbonate is added in solution B to make it pH=1, and 30g of sodium sulfate is added to impel the precipitation of sodium fluoroaluminate completely, and the stirring time is 1.5h to obtain 100g of cryolite and solution C; the amount of sodium carbonate is that of calcium sulfate ...
Embodiment 3
[0043] The applicant provides a method for reclaiming cryolite, calcium carbonate and sodium sulfate from calcium fluoride sludge resources, the specific steps are as follows:
[0044] (1) Add sulfuric acid to 1000 g of calcium fluoride sludge to adjust its pH=3. When using sulfuric acid to acidify the sludge, the time is 2 hours; the calcium existing in the form of calcium hydroxide and calcium carbonate in the sludge is converted into sulfuric acid Calcium, to obtain solution A.
[0045] (2) Add 500g of sodium aluminate solid in solution A, and carry out stirring and leaching to convert calcium fluoride into fluoaluminic acid and calcium sulfate precipitation S1 and solution B;
[0046] (3) Add sodium carbonate to solution B to make it pH=2, and add 50g of sodium sulfate to promote the complete precipitation of sodium fluoroaluminate, and stir for 2 hours to obtain 100g of cryolite and solution C; the amount of sodium carbonate is 1.5 of calcium sulfate times.
[0047] (4)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com