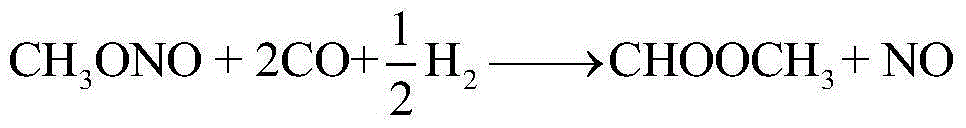Method for separating dimethyl carbonate
A technology for dimethyl carbonate and methyl formate, applied in the field of separation of dimethyl carbonate, can solve the problems of low ethylene glycol extraction efficiency, increased circulating amount of mixed liquid, and small amount of effectively separated DMC.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
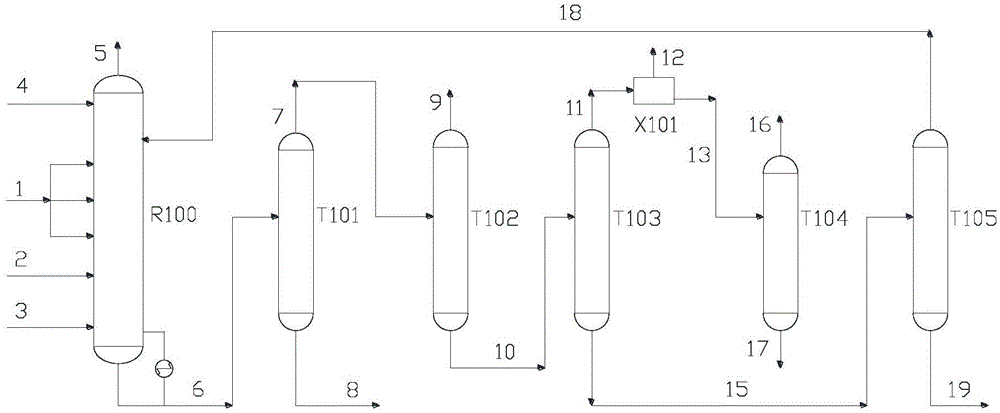
[0040] Oxygen is fed to 1, and the coupling unit is recycle gas (containing methanol, dimethyl carbonate, methyl formate, methyl nitrite, CO, NO and N 2 ) and supplemented NO stream 2, CO feed 3 and methanol feed 4 are sent to the oxidative esterification reactor R100 for oxidative esterification, and the gaseous methyl nitrite stream 5 is obtained at the top of the tower, and the tower still obtains methyl nitrite containing The stream 6 of ester, dimethyl carbonate, methyl formate, methanol, nitric acid and water; the stream 6 is sent to the weight-removing tower T101 for separation, and the mixture containing methyl nitrite, dimethyl carbonate, and methyl formate is obtained at the top of the tower and the first light component stream 7 of methanol, obtain the first heavy component stream 8 containing nitric acid and water in the tower still; stream 8 is sent to the nitric acid concentration unit (not involved in this patent) and recycles after thickening, and stream 7 is se...
Embodiment 2
[0051] The embodiment is the same as [Example 1], except that the feed composition and the process parameters of each tower are different.
[0052] Oxygen stream 1 flow rate 2500Nm 3 / h, the purity is 99.8%, the rest is N 2 , Feeding in three stages. Coupling unit to recycle gas (containing methanol, dimethyl carbonate, methyl formate, methyl nitrite, CO, NO and N 2 ) and the flow rate of logistics 2 supplemented with NO is 80000Nm 3 / h, the composition is methanol 5%, dimethyl carbonate 0.5%, methyl formate 0.45%, methyl nitrite 8%, CO 5%, NO 15%, and the rest is N 2 , CO feed 3 flow rate is 9800Nm 3 / h, purity 99.5%, H 2 500ppm, and methanol feed 4 flow rate is 12500kg / h, purity 99.9%.
[0053] The reaction temperature of the oxidative esterification reactor is 83° C., and the reaction pressure is 0.6 MPag.
[0054] The theoretical plate number of the weight removal tower is 60, the operating pressure at the top of the tower is 500kPaG, and the temperature at the top ...
Embodiment 3
[0062] The embodiment is the same as [Example 1], except that the feed composition and the process parameters of each tower are different.
[0063] Oxygen stream 1 flow rate 2500Nm 3 / h, the purity is 99.8%, the rest is N 2, Feeding in three stages. Coupling unit to recycle gas (containing methanol, dimethyl carbonate, methyl formate, methyl nitrite, CO, NO and N 2 ) and the flow rate of logistics 2 supplemented with NO is 140000Nm 3 / h, the composition is methanol 10%, dimethyl carbonate 0.12%, methyl formate 0.1%, methyl nitrite 5%, CO 15%, NO 8%, and the rest is N 2 , CO feed 3 flow rate is 9880Nm 3 / h, purity 99.5%, H 2 500ppm, and methanol feed 4 flow rate is 15500kg / h, purity 99.9%.
[0064] The reaction temperature of the oxidative esterification reactor is 30° C., and the reaction pressure is 0.1 MPag.
[0065] The theoretical plate number of the weight-removing tower is 30, the operating pressure at the top of the tower is 150kPaG, and the temperature at the to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com