Preparation method of MOFs with pi-activation catalytic action
A catalyst and ligand technology, applied in the field of heterogeneous catalytic materials, can solve the problems of low utilization rate of noble metal silver atoms, impossibility of alkyne π-activation, difficult separation and purification, etc., to achieve easy large-scale application and improve activity And catalytic conversion number, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
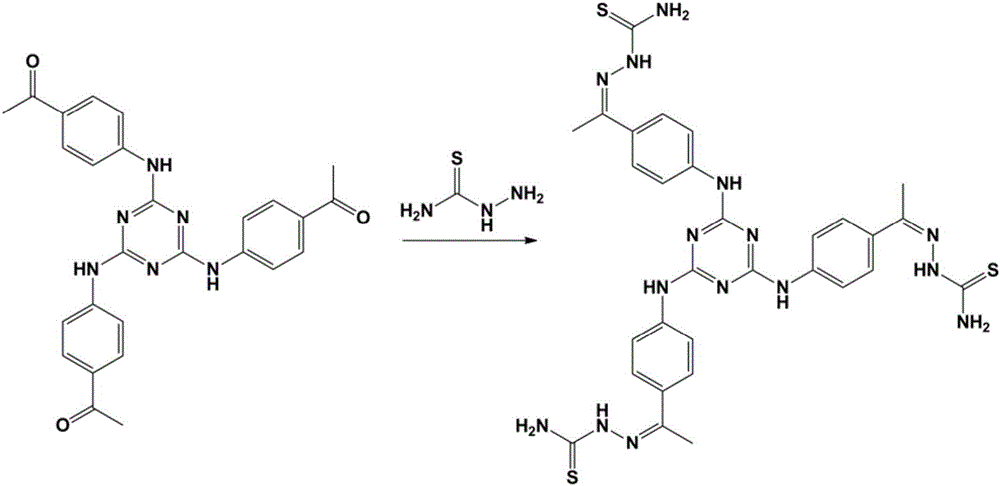
[0038] Mix 2,4,6-tris(4-acetyl-phenylamino)-1,3,5-triazine (4.81g, 10mmol) and thiosemicarbazide (2.27g, 25mmol) into 40mL of ethanol, reflux at 80°C for 10h , filtered with suction, rinsed with methanol, and dried in vacuo to obtain light yellow solid ligand TzNPAMeTs. Yield: 3.5 g, 50%. 1H-NMR[400MHz,DMSO-d6,ppm]:10.15(s,1H,-NH),9.65(s,1H,Ar-NH-),8.25(s,1H,-NH2),7.98-7.77(m ,5H,Ar-H and-NH2).13C-NMR[500MHz,DMSO-d6,ppm]: 178.59,171.97,147.85,140.72,131.27,126.95,119.60,13.78.ESI-MS:m / z 700.20.
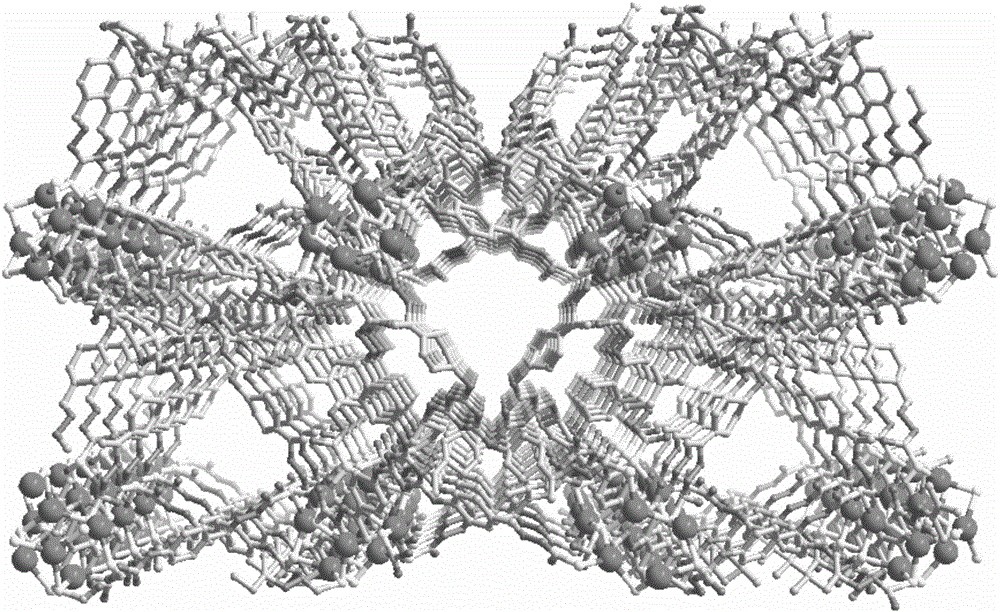
[0039] In a thin glass test tube, the ligand TzNPAMeTs (0.01mmol, 7.0mg) was added to 1mL N,N-dimethylformamide solution to make the lower layer solution, and then the metal silver salt AgBF 4 (0.03mmol, 5.8mg) was added to 1mL of acetonitrile to make the upper layer solution, the middle layer was 1mL of a mixed solution of N,N-dimethylformamide and acetonitrile with a volume ratio of 1:5. , after about 4 weeks, colorless to pale yellow crystals precipitated, which was the catalyst...
Embodiment 2
[0041] Mix thiosemicarbazide (2.73g, 30mmol) and 2,4,6-tris(4-acetyl-phenylamino)-1,3,5-triazine (4.81g, 10mmol) into 50mL of ethanol, reflux at 85°C for 12h , filtered with suction, rinsed with methanol, and dried in vacuo to obtain light yellow solid ligand TzNPAMeTs. Yield: 6.3 g, 90%. 1H-NMR[400MHz,DMSO-d6,ppm]:10.15(s,1H,-NH),9.65(s,1H,Ar-NH-),8.25(s,1H,-NH2),7.98-7.77(m ,5H,Ar-H and-NH2).13C-NMR[500MHz,DMSO-d6,ppm]: 178.59,171.97,147.85,140.72,131.27,126.95,119.60,13.78.ESI-MS:m / z 700.20.
[0042] In a thin glass test tube, the ligand TzNPAMeTs (0.01mmol, 7.0mg) was added to 1mL N,N-dimethylformamide solution to make the lower layer solution, and then the metal silver salt AgBF 4 (0.03mmol, 5.8mg) was added to 1mL of acetonitrile to make the upper layer solution, the middle layer was 1mL of a mixed solution of N,N-dimethylformamide and acetonitrile with a volume ratio of 5:1. , after about 4 weeks, colorless to pale yellow crystals precipitated, which was the catalyst...
Embodiment 3
[0044] Mix thiosemicarbazide (3.18g, 35mmol) and 2,4,6-tris(4-acetyl-phenylamino)-1,3,5-triazine (4.81g, 10mmol) into 60mL of ethanol, reflux at 90°C for 15h , filtered with suction, rinsed with methanol, and dried in vacuo to obtain light yellow solid ligand TzNPAMeTs. Yield: 6.7 g, 95%. 1H-NMR[400MHz,DMSO-d6,ppm]:10.15(s,1H,-NH),9.65(s,1H,Ar-NH-),8.25(s,1H,-NH2),7.98-7.77(m ,5H,Ar-H and-NH2).13C-NMR[500MHz,DMSO-d6,ppm]: 178.59,171.97,147.85,140.72,131.27,126.95,119.60,13.78.ESI-MS:m / z700.20.
[0045] In a thin glass test tube, the ligand TzNPAMeTs (0.01mmol, 7.0mg) was added to 1mL N,N-dimethylformamide solution to make the lower layer solution, and then the metal silver salt AgBF 4 (0.03mmol, 5.8mg) was added to 1mL of acetonitrile to make the upper layer solution, the middle layer was 1mL of a mixed solution of N,N-dimethylformamide and acetonitrile with a volume ratio of 1:1. , after about 4 weeks, colorless to pale yellow crystals precipitated, which was the catalyst ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com