Pharmaceutical composition containing sugar reducing medicine, aspirin and folic acid
An aspirin and composition technology, which is applied in the directions of drug combinations, medical preparations containing active ingredients, active ingredients of heterocyclic compounds, etc., can solve the problems of ignoring the early intervention of diabetic complications and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
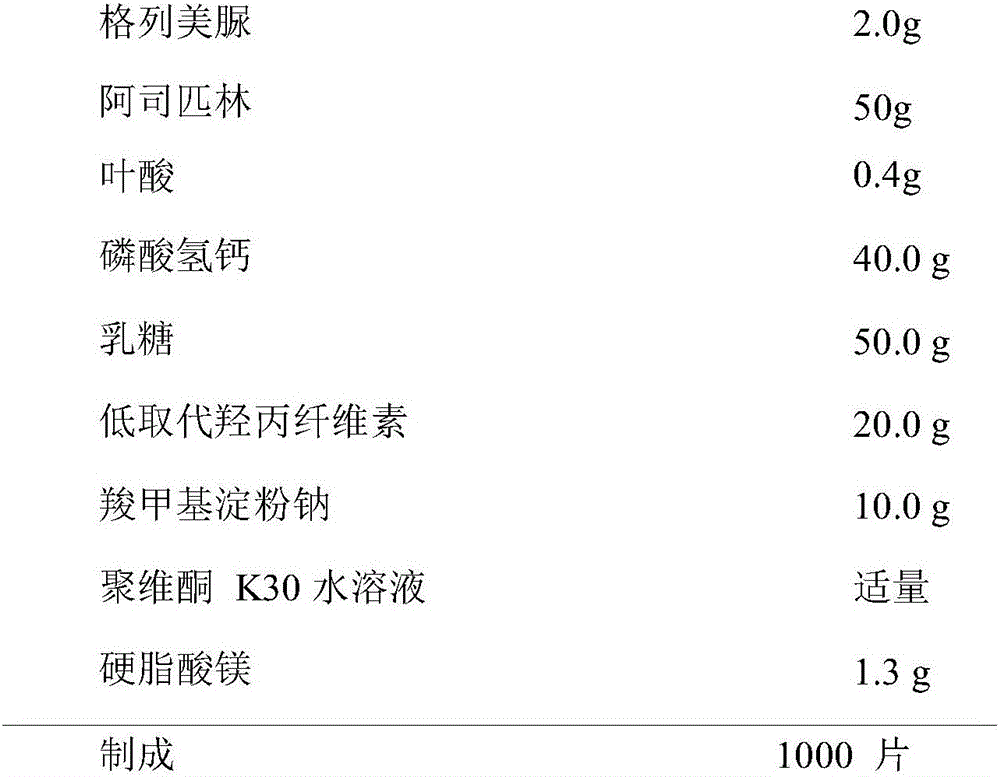
[0052] Example 1 Preparation of Compound Glimepiride / Aspirin / Folic Acid Tablets (1000 Tablets)
[0053] formula:
[0054]
[0055] Preparation:
[0056] The raw and auxiliary materials are crushed through an 80-mesh sieve, and dried for later use. Take the prescribed amount of glimepiride, aspirin, and folic acid and mix them uniformly according to the method of equal increase, add sodium carboxymethyl starch and low-substituted hydroxypropyl cellulose respectively according to the prescription amount, and mix uniformly according to the method of equal increase to obtain powder A ,spare. Dibasic calcium phosphate and lactose were passed through a 100-mesh sieve and dried at 75°C for 2 hours. Mix powder A with the mixed calcium hydrogen phosphate and lactose in equal increments. Add appropriate amount of povidone K30 aqueous solution binder to make soft material, granulate with 20-mesh sieve, dry at 40-45°C; dry granules are granulated with 20-mesh sieve. Add 1% magnes...
Embodiment 2~17
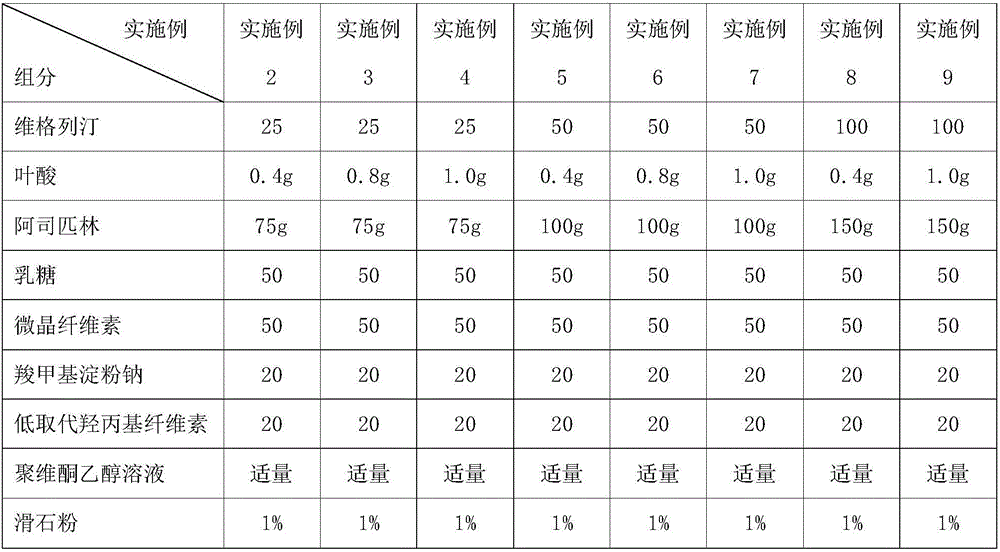
[0057] Examples 2-17 : the preparation of the compound tablet of different content proportioning (1000 pieces quantity)
[0058] The preparation method of Examples 2-17 is the same as that of Example 1, and the granules obtained according to the formula shown in Table 1 are made into tablets.
[0059] Table 1 embodiment 2~9 tablet formula composition
[0060]
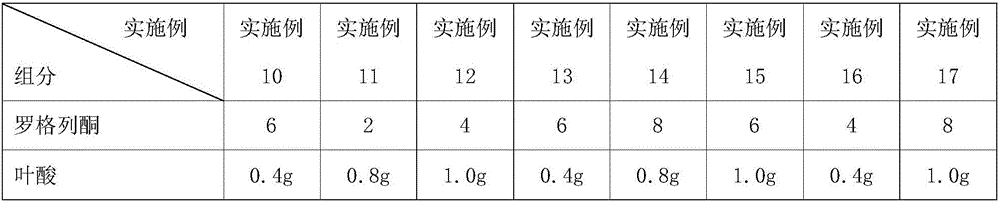
[0061] Continuation of table 1 embodiment 10~17 tablet formula composition
[0062]
[0063]
Embodiment 18
[0064] Example 18 Preparation of Sitagliptin / Aspirin / Folic Acid Capsules (1000 Capsules)
[0065] formula:
[0066]
[0067] Preparation method: crush the raw and auxiliary materials through an 80-mesh sieve, and dry for later use. Take 50g of sitagliptin, 75g of aspirin, and 0.8g of folic acid and mix them uniformly according to the equal increase method, add calcium hydrogen phosphate, lactose, sodium carboxymethyl starch and low-substituted hydroxypropyl cellulose respectively, and mix evenly according to the equal increase method , use povidone ethanol solution to make soft material, granulate with 20 mesh sieve, dry at 40°C for about 2 hours, granulate with 18 mesh sieve, control the water content of the granules to 2-3%, mix the dried granules with micro-powder silica gel Uniform, semi-finished products are tested, the content is measured, and filled into hollow capsules. Pay attention to avoiding light during the preparation process, and the prepared capsules nee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com