Method for separating boron and iron from ludwigite
A boronite and ferroboron technology, which is applied in the fields of borax, boric acid and high-grade iron concentrate, preparation of sodium metaborate/perborate, and boronite and ferroboron separation, which can solve the problem of high energy consumption, equipment corrosion, and difficulty in waste acid. To deal with other problems, to achieve the effect of improving resource utilization, easy operation control, and improving utilization value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A method for separating boron-iron from boron-containing iron ore, said boron-iron ore is boron-iron ore powder in a certain place in Liaoning, and the composition of this boron-iron ore is:
[0050] components B 2 o 3
SiO 2
CaO MgO al 2 o 3
TF Na 2 o
Contentwt% 5.88 4.23 1.62 11.24 0.47 52.98 0.18
[0051] The method comprises the steps of:
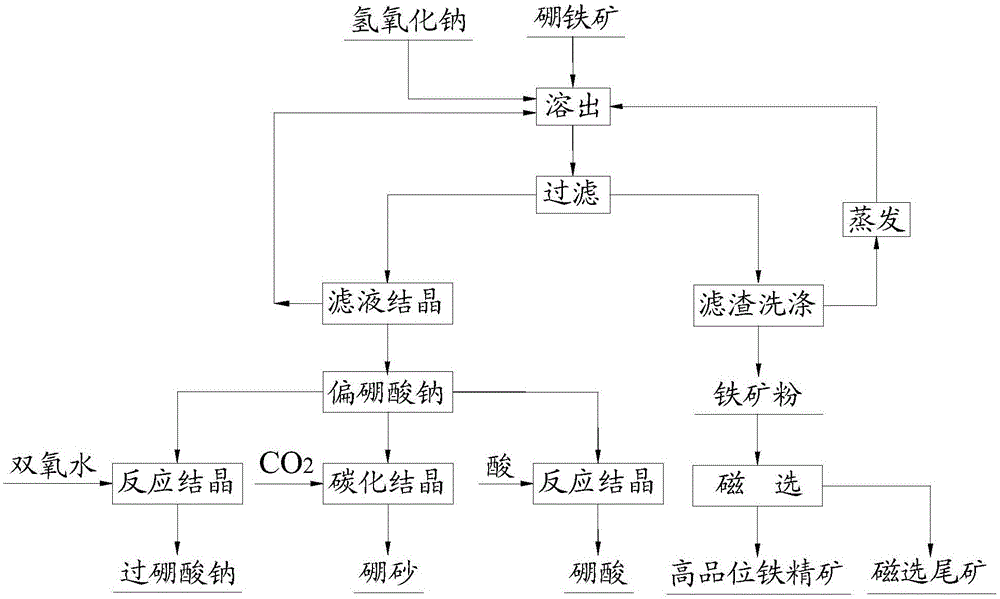
[0052] (1) NaOH solution with a mass fraction of 25%, according to the mass ratio of NaOH solution: boronite powder = 4:1, mix the ingredients evenly, place in the reaction kettle, heat up to 180°C, react for 2h, cool to 90°C, feed The slurry is filtered to obtain the iron ore powder filter residue and the eluate containing sodium metaborate;
[0053] (2) Add calcium oxide to the eluate containing sodium metaborate, stir at 100°C for 2 hours to remove impurities, filter, evaporate and concentrate the filtrate, cool and crystallize at 25-30°C for 6 hours, and filter to ob...
Embodiment 2
[0058] A method for the separation of boronite or boron and iron, the composition of the boronite is:
[0059] components B 2 o 3
SiO 2
CaO MgO Al 2 o 3
TF Na 2 o
[0060] Contentwt% 4.24 6.90 2.31 13.98 3.32 45.16 0.12
[0061] The method comprises the steps of:
[0062] (1) NaOH with a mass fraction of 25%, mix the ingredients according to the mass ratio of NaOH solution: boronite powder = 1:1, place in the reaction kettle, heat up to 160°C, react for 2h, cool to 90°C, and make the slurry Obtain iron ore powder filter cake and the eluate containing sodium metaborate through filtration;
[0063] (2) Add calcium oxide to the eluate containing sodium metaborate, stir at 100°C for 2 hours to remove impurities, filter, evaporate and concentrate the filtrate, cool and crystallize at 25-30°C for 6 hours, and filter to obtain sodium metaborate;
[0064] (3) Dissolve sodium metaborate in water, add hydrogen perox...
Embodiment 3
[0068] A method for the separation of boronite and boron iron, the composition of the boronite is the same as that of Example 1, and the method may further comprise the steps:
[0069] (1) NaOH with a mass fraction of 35%, according to the mass ratio of NaOH solution: boronite powder = 4:1, mix the ingredients evenly, place in the reaction kettle, heat up to 160°C, react for 2h, cool to 90°C with water, The slurry is filtered to obtain the iron ore powder filter cake and the eluate containing sodium metaborate;
[0070] (2) Add calcium oxide to the eluate containing sodium metaborate, stir at 100°C for 2 hours to remove impurities, filter, evaporate and concentrate the filtrate, cool and crystallize at 25-30°C for 6 hours, and filter to obtain sodium metaborate;
[0071] (3) Dissolve sodium metaborate in water, and introduce CO 2 , react at 30-40°C for 3-4 hours, cool to 20-25°C to crystallize, and obtain borax;
[0072] (4) The iron ore powder filter residue of step (1) is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com