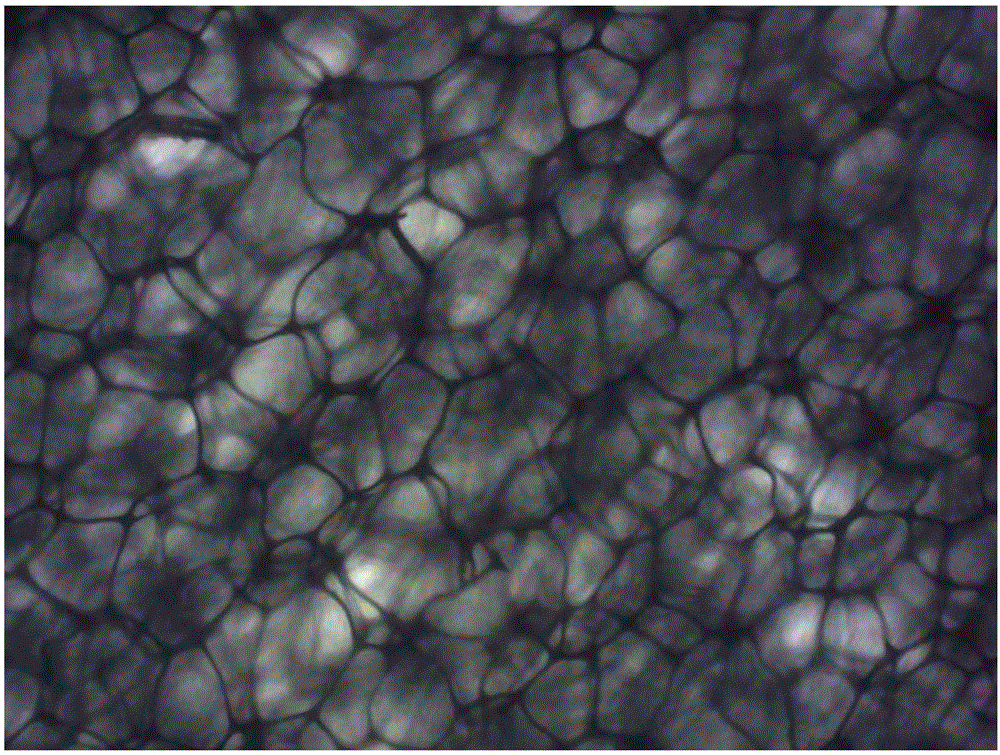
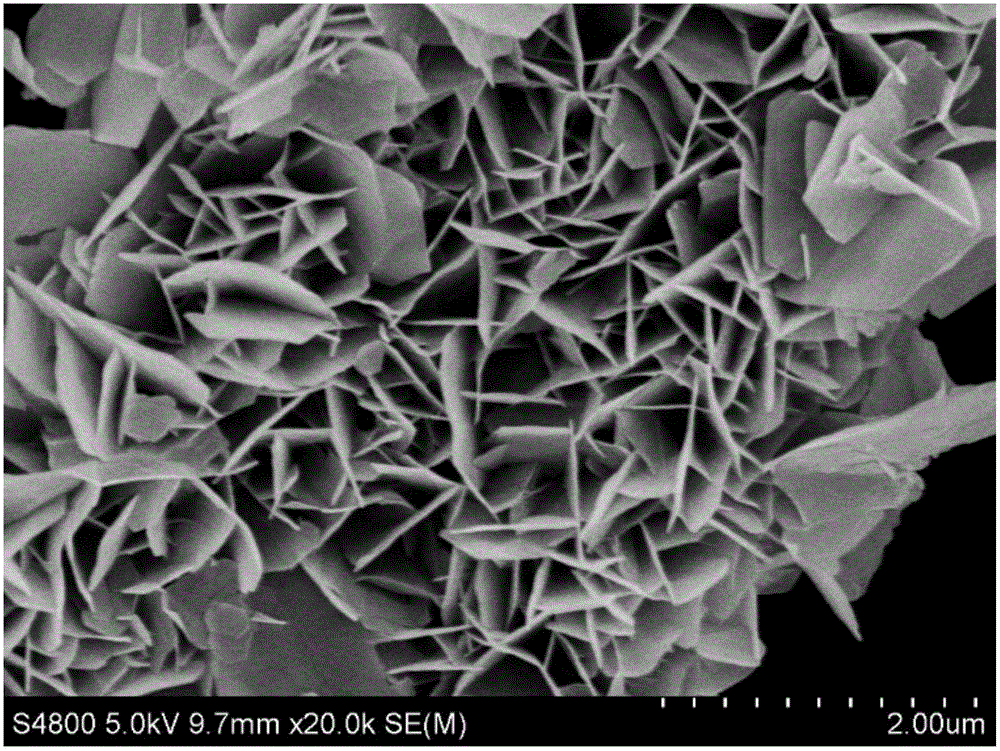
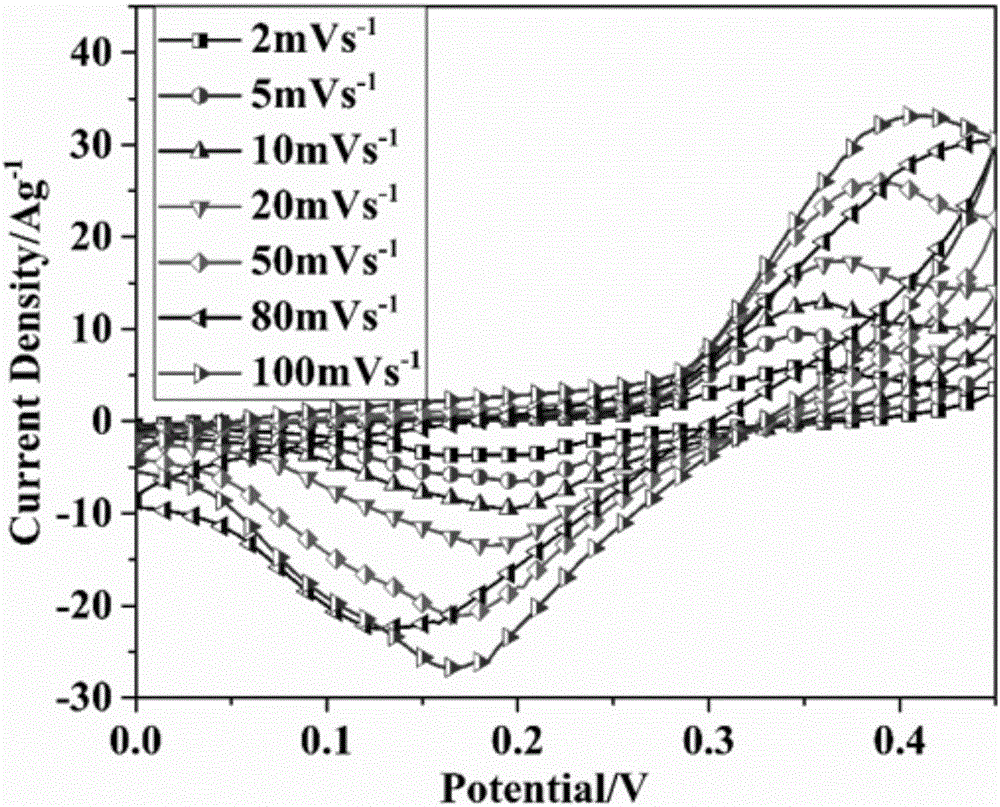
Flexible self-supported porous carbon @ layered bimetallic hydroxide composite material, its preparation method and application
A layered bimetallic, hydroxide technology, applied in the field of composite materials, can solve the problems of graphene agglomeration, complex process, reduced process efficiency, etc., achieve high specific capacitance value and rate characteristics, simple preparation process, and facilitate rapid transmission. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Take a block of melamine sponge and place it in a tube furnace, pass in argon gas as a protective atmosphere, raise the temperature to 800°C at a heating rate of 2°C / min, carbonize for 2h, then cool down naturally to room temperature, and take it out for later use. Weigh 0.175g Ni(NO 3 ) 2 ·6H 2 O, 0.088gCo(NO 3 ) 2 ·6H 2 O. 0.126g of urea was dissolved in 60mL of deionized water to obtain a metal salt solution. Weigh 24mg of porous carbon and immerse in the metal salt solution, transfer to a 100mL hydrothermal reaction kettle, and react at 100°C for 24 hours. After cooling to room temperature, the product was collected by suction filtration, washed with excess deionized water until neutral, and dried in a vacuum oven at 60°C to obtain the product. The obtained product was used as the active component of the supercapacitor electrode, and 2 mg of flexible self-supporting porous carbon@layered double metal hydroxide composite material was clipped and clamped by the ...
Embodiment 2
[0033] Take a block of melamine sponge and place it in a tube furnace, pass in argon gas as a protective atmosphere, raise the temperature to 800°C at a heating rate of 2°C / min, carbonize for 2h, then cool down naturally to room temperature, and take it out for later use. Weigh 0.175g Ni(NO 3 ) 2 ·6H 2 O, 0.076gMn(NO 3 ) 2 4H 2O. 0.126g of urea was dissolved in 60mL of deionized water to obtain a metal salt solution. Weigh 24mg of porous carbon and immerse in the metal salt solution, transfer to a 100mL hydrothermal reaction kettle, and react at 100°C for 24 hours. After cooling to room temperature, the product was collected by suction filtration, washed with excess deionized water until neutral, and dried in a vacuum oven at 60°C to obtain the product. The obtained product was used as the active component of the supercapacitor electrode, and 2 mg of flexible self-supporting porous carbon@layered double metal hydroxide composite material was clipped and clamped by the el...
Embodiment 3
[0035] Take a block of melamine sponge and place it in a tube furnace, pass in argon gas as a protective atmosphere, raise the temperature to 800°C at a heating rate of 2°C / min, carbonize for 2h, then cool down naturally to room temperature, and take it out for later use. Weigh 0.175g Co(NO 3 ) 2 ·6H 2 O, 0.076gMn(NO 3 ) 2 4H 2 O. 0.126g of urea was dissolved in 60mL of deionized water to obtain a metal salt solution. Weigh 24mg of porous carbon and immerse in the metal salt solution, transfer to a 100mL hydrothermal reaction kettle, and react at 100°C for 24 hours. After cooling to room temperature, the product was collected by suction filtration, washed with excess deionized water until neutral, and dried in a vacuum oven at 60°C to obtain the product. The obtained product was used as the active component of the supercapacitor electrode, and 2 mg of flexible self-supporting porous carbon@layered double metal hydroxide composite material was clipped and clamped by the e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com