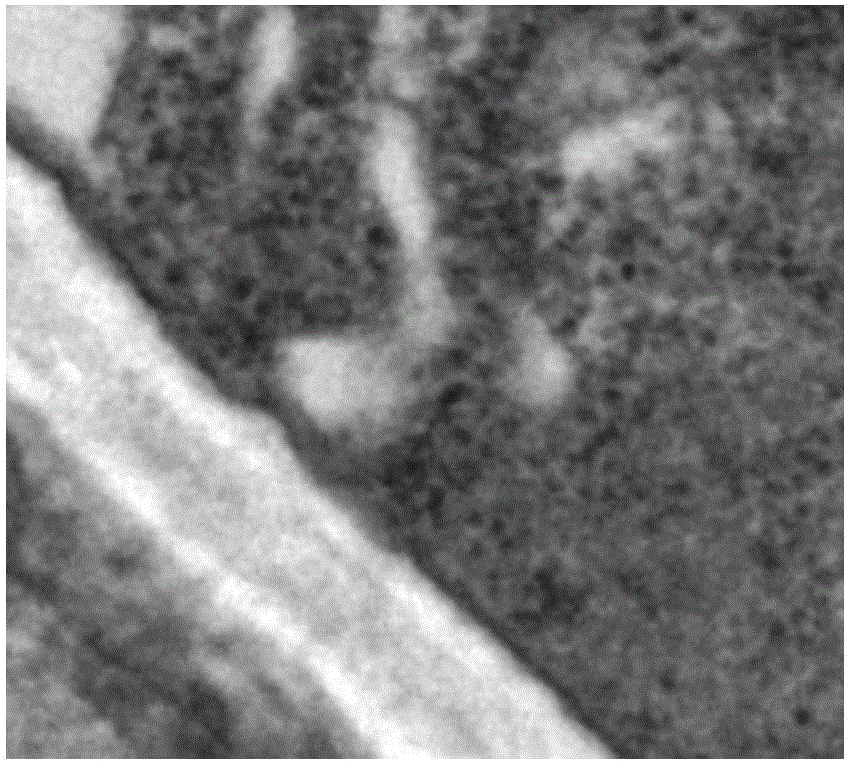
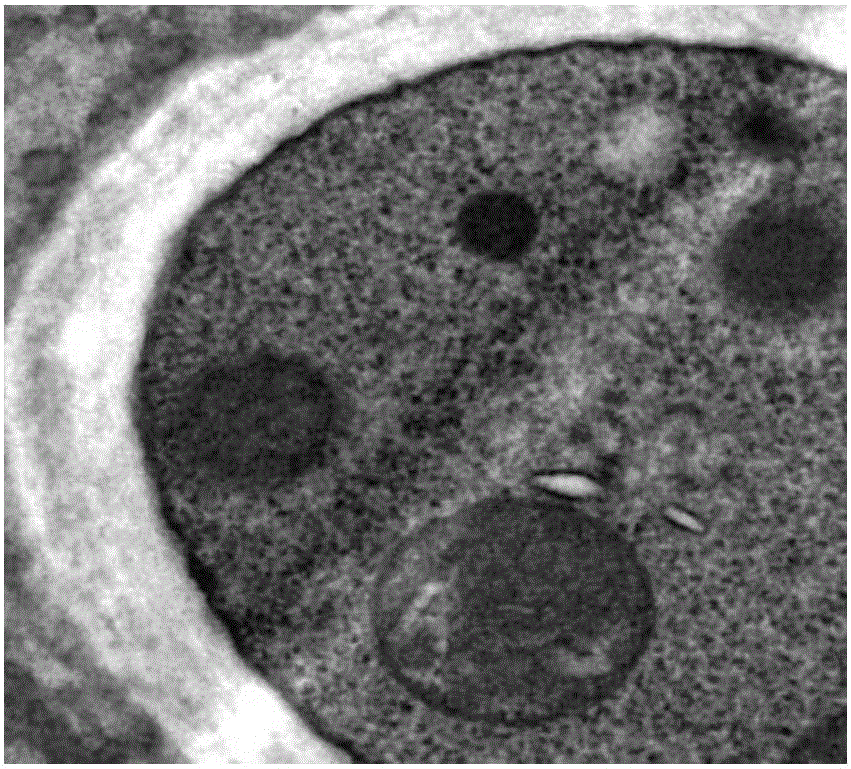
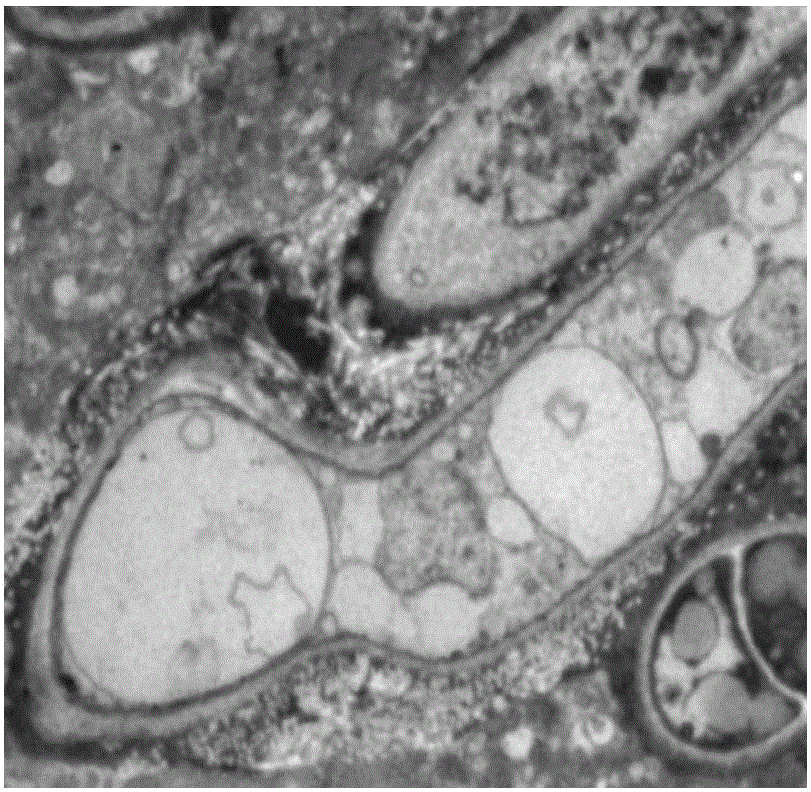
Application of cinnamaldehyde in preparation of drugs for targeted therapy of drug-resistant aspergillus infection
A technology of drug aspergillus and targeted therapy, applied in the field of medicine, can solve problems such as poor prognosis and difficult clinical treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 removes patchouli ketone for comparative example 1, and others remain unchanged.
[0031] The tested invasive and drug-resistant Aspergillus strains came from the Mycology Department of the Second Hospital of Hebei Medical University. Direct microscopic examination of sputum specimens showed a large number of thick mycelium separated by branches at 45 degrees. , after identification and drug susceptibility test as invasive drug-resistant Aspergillus fumigatus and Aspergillus flavus, the results are shown in Table 1.
[0032] Table 1 Antifungal drugs against invasive drug-resistant Aspergillus MIC mgml -1 value
[0033] Invasive resistant Aspergillus strains n Fluconazole Itraconazole Amphotericin B Voriconazole Ketoconazole Flucytosine Aspergillus fumigatus 30 16-64 8-16 1-8 1-4 64 64 Aspergillus flavus 30 8-32 4-16 8-32 4-16 64 64
[0034] According to the method described in Experimental Example 1 discl...
Embodiment 2
[0038] In order to further develop the clinical effect of cinnamaldehyde used alone, the present invention proposes to include cinnamic aldehyde with hydroxypropyl-β-cyclodextrin to obtain cinnamic aldehyde monomer clathrate. The cinnamon aldehyde monomer clathrate can be further prepared into oral and injection dosage forms through conventional techniques in the field of pharmaceutical technology. The cinnamon aldehyde monomer clathrate is prepared from the following components in parts by weight: 10-60 cinnamaldehyde and 20-80 hydroxypropyl-β-cyclodextrin. The hydroxypropyl-β-cyclodextrin is 2-hydroxypropyl-β-cyclodextrin. Embodiment 2 cinnamic aldehyde monomer clathrate of the present invention
[0039] Composition: Cinnamaldehyde 1.25g (50 parts by weight)
[0040] 2-hydroxypropyl-β-cyclodextrin 1.25g (50 parts by weight)
[0041] Preparation:
[0042] 1) Add 2-hydroxypropyl-β-cyclodextrin to 80°C deionized distilled water, stir to dissolve, then cool down to 20°C to p...
Embodiment 3
[0044] 3) Filter the refrigerated liquid obtained in step 2), wash the solid matter with a small amount of distilled water, dry it in vacuum at a low temperature of 5°C for 24 hours, grind it, sieve it through 80 mesh, wash it with ethyl acetate three times, and dry it in the air to obtain white Loose cinnamaldehyde monomer inclusion compound. Embodiment 3 cinnamic aldehyde monomer clathrate of the present invention
[0045] Composition: Cinnamaldehyde 1g (10 parts by weight)
[0046] 2-hydroxypropyl-β-cyclodextrin 2g (20 parts by weight)
[0047] Preparation method: with embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com