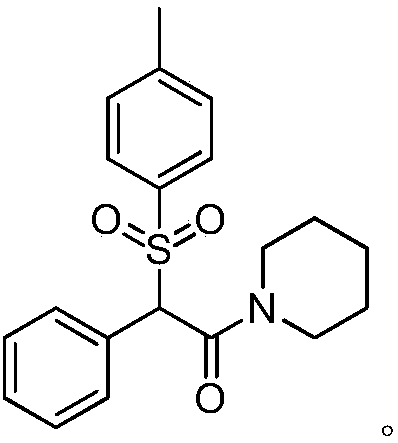
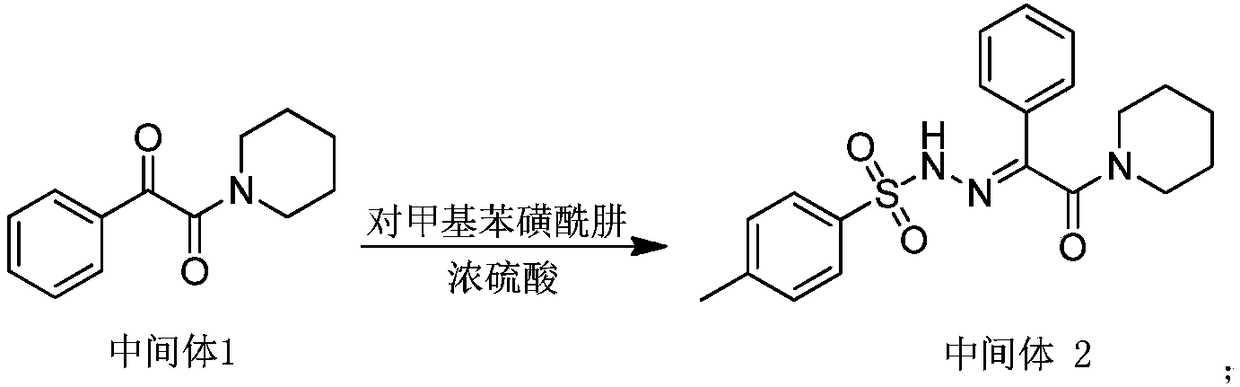
Impurity g produced by the preparation process of methylphenidate hydrochloride and its purification method and use
A technology of methylphenidate hydrochloride and preparation process, applied in the field of impurity G and purification thereof, can solve problems such as no records or reports of impurities, and achieve the effects of easy operation, high reaction yield and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] 1. Preparation of crude product of impurity G
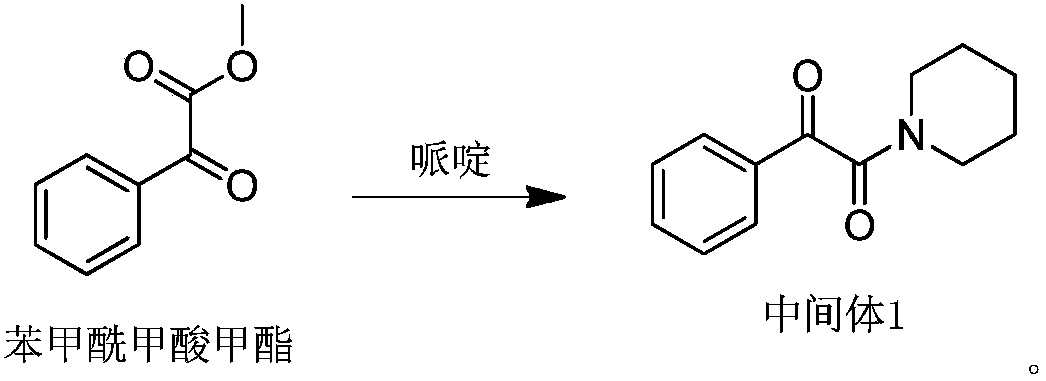
[0061] a), the preparation of intermediate 1
[0062] A. Synthetic reaction equation:
[0063]
[0064] B. Process description
[0065] Add 1.0Lg of methanol into a dry and clean 3L three-necked flask, start stirring, add 0.37kg of piperidine and 0.59kg of methyl benzoylformate in sequence, heat up to 50-55°C and keep it warm for 2 hours, then monitor the reaction process by TLC (GF254 Silica gel plate; developing agent: ethyl acetate / petroleum ether = 1 / 2), stop the reaction when the raw material point disappears, put the material into a clean and dry sesame cake and slowly cool down to 0-5°C to fully separate out the solid, filter, filter The cake was stirred and washed twice with methanol pre-cooled to -5 ~ 0°C, each time 0.5kg, and air-dried at 60°C overnight to obtain 0.65kg of intermediate 1, a white solid, which was detected by the HPLC area normalization method. The content of body 1 is 99.50%, and the yield ...
Embodiment 1
[0078] Example 1 12 times of n-heptane dissolved impurity G crude product, 6 times of methanol refining
[0079] 1) Add 40.0g of impurity G crude product and 480.0g of n-heptane to a dry and clean 1000mL three-neck flask, start stirring, raise the temperature to 95°C to dissolve the sample, filter to remove a small amount of insoluble matter, and slowly cool the filtrate to 55°C for crystallization. After the solid was precipitated, it was filtered to obtain a filter cake, and dried at 60° C. to obtain 2.8 g of a white solid.
[0080] 2) Add 2.8g of the obtained solid into a dry and clean 50mL eggplant-shaped bottle, add 16.8g of methanol, start stirring, heat to dissolve, slowly cool down to 2°C after dissolution, keep stirring for 45 minutes, filter, and dry to weight at 50°C Without subtraction, 1.3g of impurity G fine product was obtained as a white solid, and the content of impurity G fine product was 99.60% as measured by HPLC area normalization method.
[0081] 3) Deco...
Embodiment 2
[0083] Example 2 10 times the crude product of n-heptane dissolved impurity G, refined by 6 times methanol
[0084] 1) Add 40.0g of impurity G crude product and 400.0g of n-heptane to a dry and clean 1000mL three-necked flask, start stirring, raise the temperature to 80°C to dissolve the sample, filter to remove a small amount of insoluble matter, and slowly cool the filtrate to 55°C to crystallize. After the solid was precipitated, it was filtered to obtain a filter cake, which was dried at 60° C. to obtain 2.9 g of a white solid.
[0085] 2) Add 2.9g of the obtained solid into a dry and clean 50mL eggplant-shaped bottle, add 17.4g of methanol, start stirring, heat to dissolve, slowly cool down to 2°C after dissolution, keep stirring for 45 minutes, filter, and dry to weight at 50°C Without subtraction, 1.5g of impurity G fine product was obtained as a white solid, and the content of impurity G fine product was 99.20% as measured by HPLC area normalization method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com