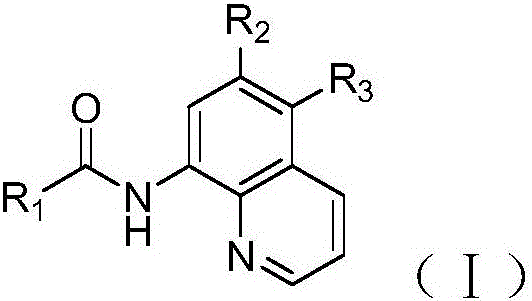
8-aminoquinoline derivative and preparation and application thereof
A technology for aminoquinoline and derivatives, which is applied in the field of 8-aminoquinoline derivatives, and can solve problems such as high cost, complicated preparation, and lack of modification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
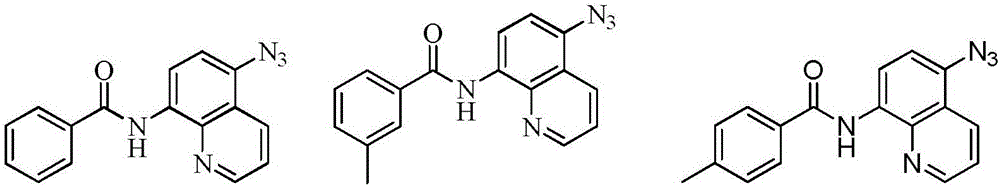
[0049] Embodiment 1: Preparation N-(5-azidoquinoline-8-amino) benzamide
[0050]
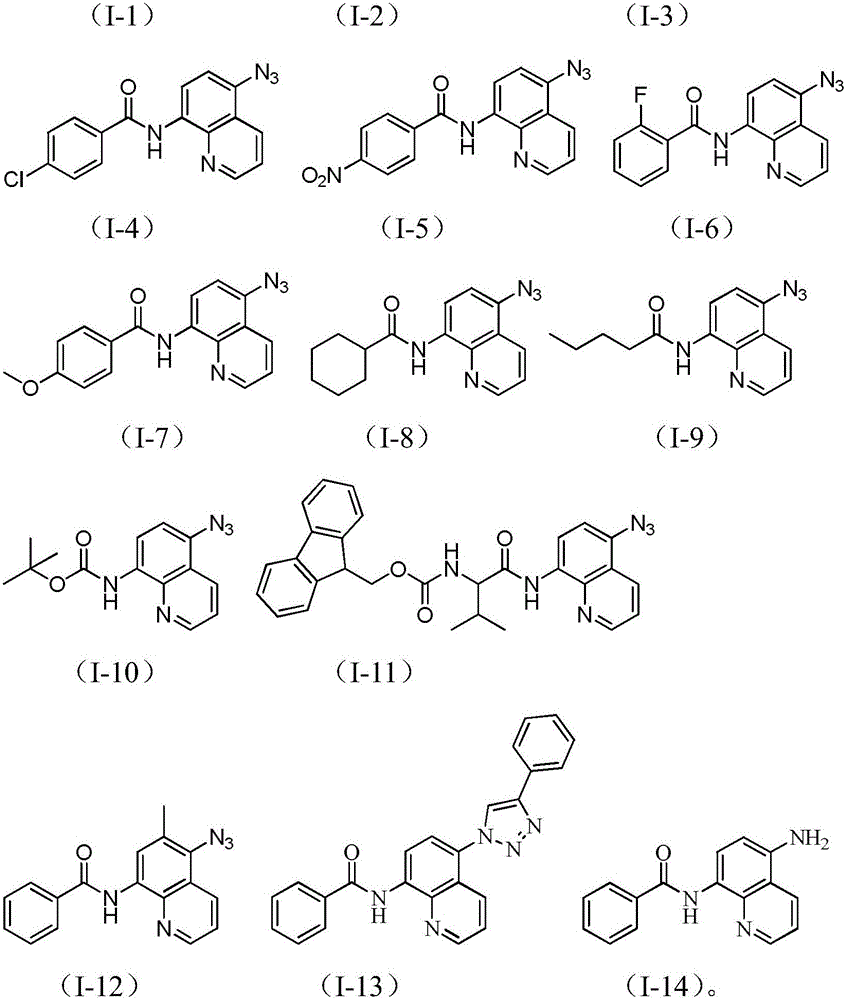
[0051] Add 0.1mmol of N-(quinoline-8amino)benzamide (II-1) to 5ml of N,N-dimethylformamide and 5ml of deionized water to make a mixed solution of 10ml, and add 0.3mmol of azide to it Sodium chloride, copper acetate 0.03mmol, 0.3mmol potassium persulfate, 0.1mmol DBU, 0.1mmol TBAB, react at 40°C for 12 hours, after the reaction, add saturated NaCl aqueous solution to the reaction solution, extract with dichloromethane, take the organic layer through Dry over anhydrous magnesium sulfate, filter, evaporate under reduced pressure at 60°C to remove the solvent, and obtain the crude compound represented by the formula (I-1). The crude compound represented by formula (I-1) was subjected to silica gel column chromatography, with a solution of ethyl acetate and petroleum ether at a volume ratio of 1:5 as the mobile phase, and the eluent with an Rf value of 0.3-0.5 was collected by TLC tracking , the ...
Embodiment 2
[0053] Embodiment 2: Preparation of N-(5-azidoquinoline-8-amino)-3-methyl-benzamide
[0054]
[0055] Add 0.1mmol N-(quinoline-8 amino)-3-methylbenzamide (II-2) to 5ml of N,N-dimethylformamide and 5ml of deionized water to make a mixed solution of 10ml, add 0.3mmol sodium azide, 0.03mmol copper acetate, 0.3mmol potassium persulfate, 0.1mmol DBU, 0.1mmol TBAB, react at 40°C for 12 hours, after the reaction, add saturated NaCl aqueous solution to the reaction solution, extract with dichloromethane, The organic layer was dried over anhydrous magnesium sulfate, filtered, and evaporated under reduced pressure at 60°C to remove the solvent to obtain the crude compound of the formula (I-2), and the crude compound of the formula (I-2) was subjected to silica gel column chromatography , using a solution of ethyl acetate and petroleum ether with a volume ratio of 1:10 as the mobile phase, TLC tracked and collected the eluent with an Rf value of 0.3-0.5, and collected the eluent obtai...
Embodiment 3
[0057] Embodiment 3: Preparation of N-(5-azidoquinoline-8-amino)-4-methyl-benzamide
[0058]
[0059]Add 0.1mmol N-(5-quinoline-8-amino)-4-methyl-benzamide (II-3) to 5ml of N,N-dimethylformamide and 5ml of deionized water to make a mixed solution of 10ml , add 0.3mmol sodium azide, 0.03mmol copper acetate, 0.3mmol potassium persulfate, 0.1mmol DBU, 0.1mmol TBAB, and react at 40°C for 12 hours. Extract with methyl chloride, take the organic layer, dry it with anhydrous magnesium sulfate, filter, evaporate under reduced pressure at 60°C to remove the solvent, and obtain the crude compound shown in the formula (I-3), and carry out the crude product of the compound shown in the formula (I-3) Column chromatography, using a solution of ethyl acetate and petroleum ether with a volume ratio of 1:5 as the mobile phase, TLC tracking and collecting the eluent with an Rf value of 0.3-0.5, and the collected eluent was removed from the solvent under reduced pressure. After drying, 20 mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com