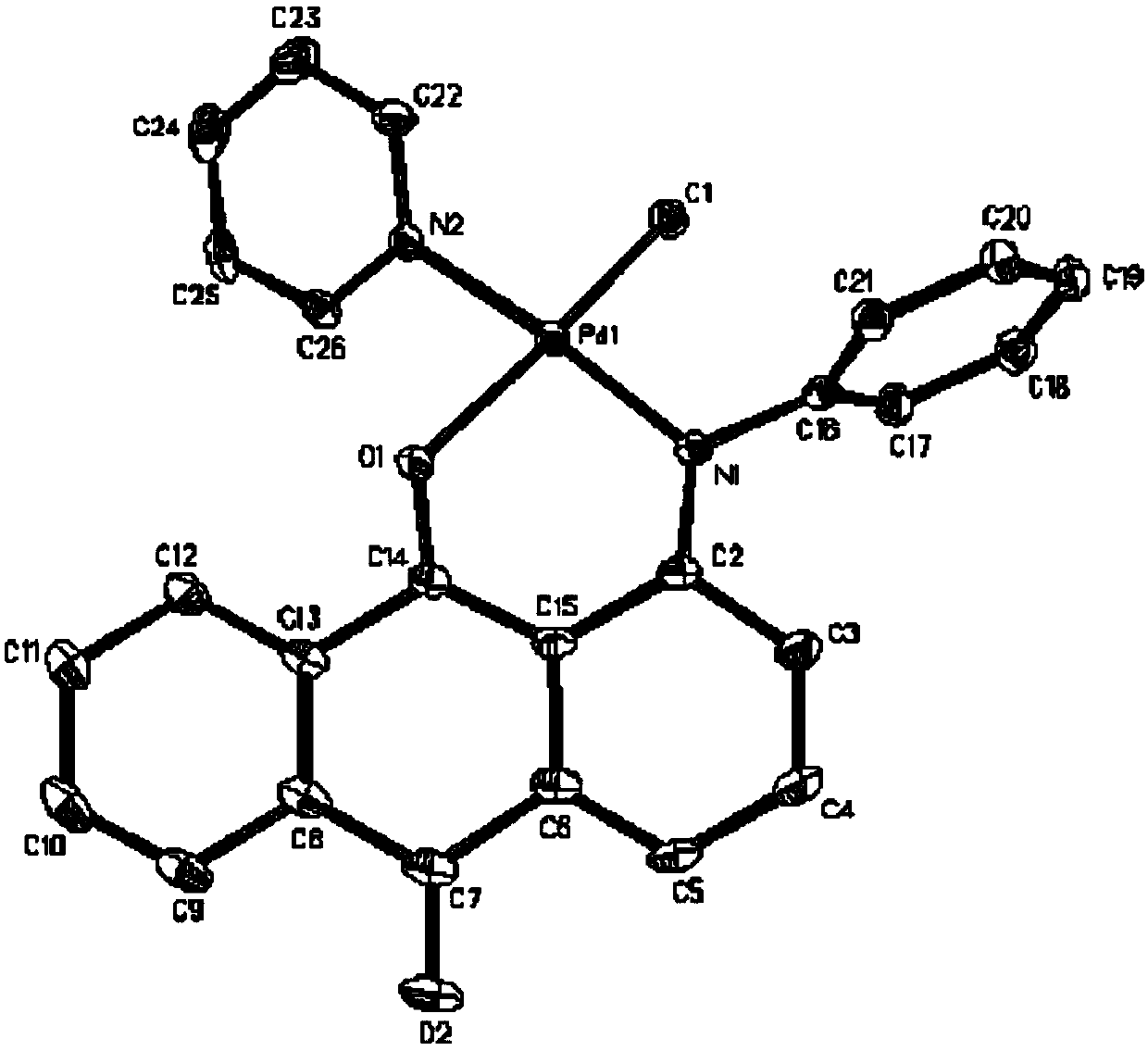
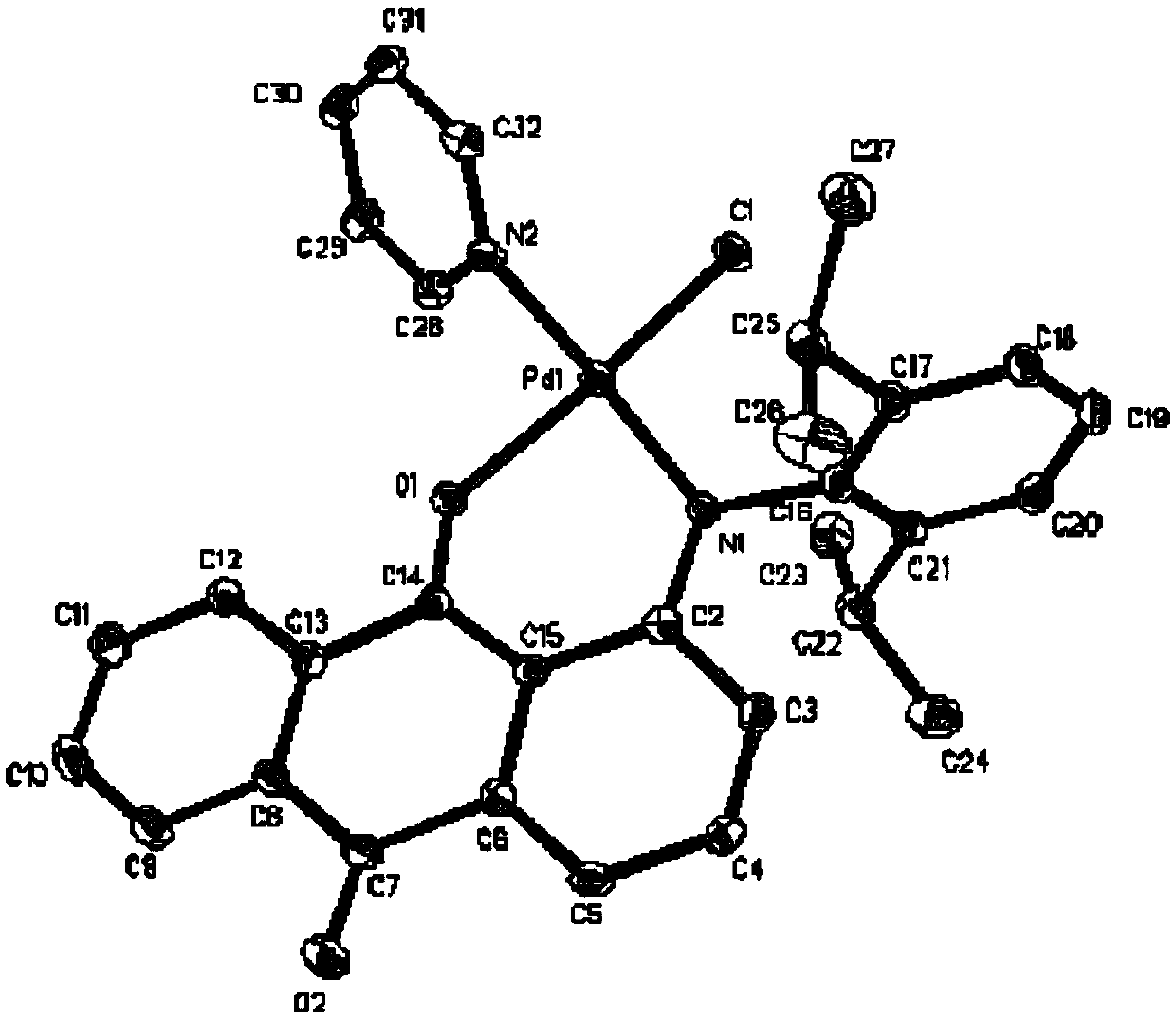
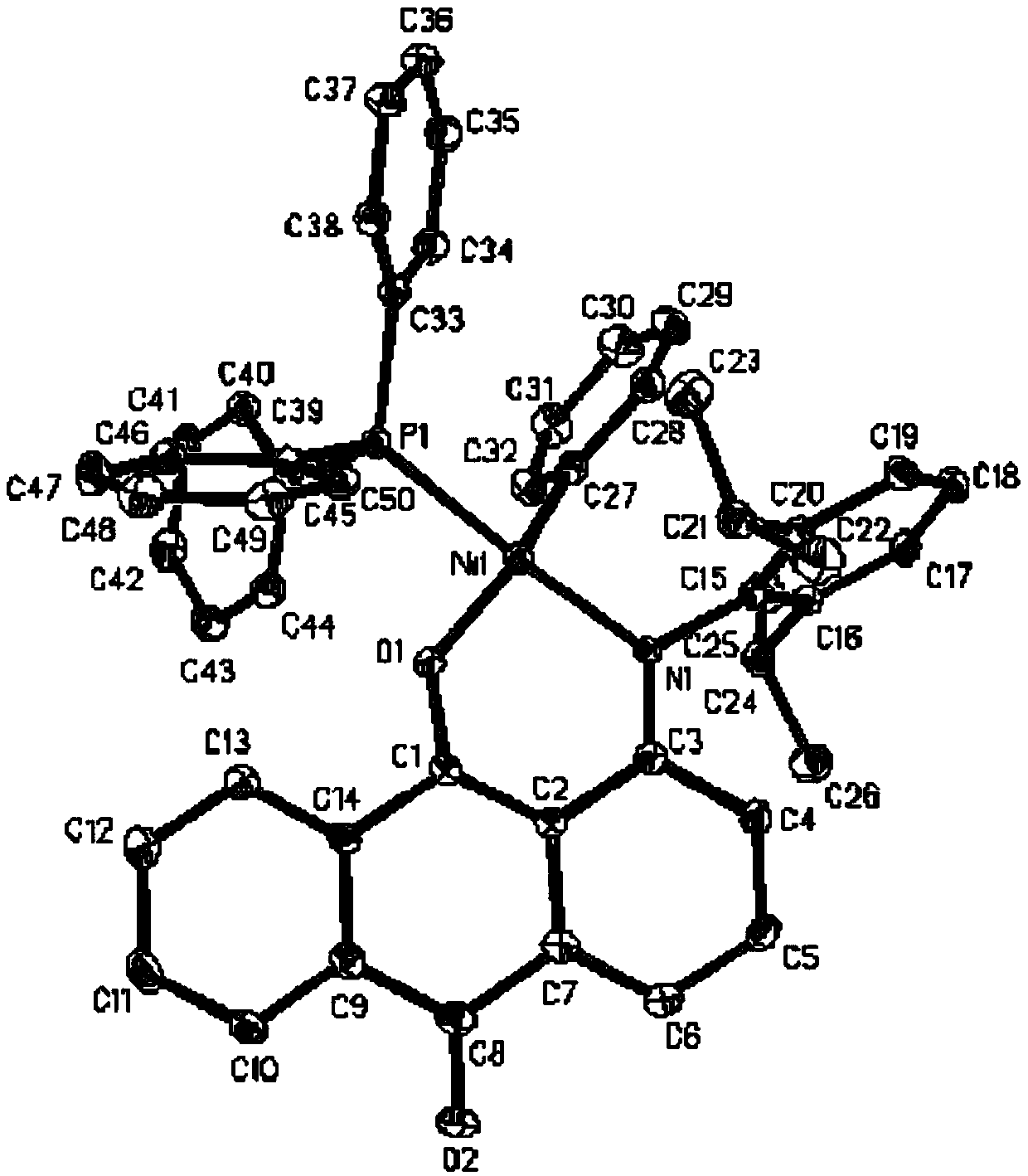
Anilinoanthraquinone post-transition metal complexes and their preparation methods and applications
A transition metal, aniline anthracene technology, applied in the field of olefin catalysis, can solve the problems of low polymerization activity, not too high activity, and no research on the polymerization ability of polar monomers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] A preparation method of aniline anthraquinone transition metal complex, including the following steps:
[0069] (1) Dissolve 1-chloroanthraquinone and aniline with a molar ratio of 1:1.5 in toluene, and then add a catalytic system, the catalytic system is bis(dibenzylideneacetone) palladium, large hindered phosphino dicene Iron and Cs 2 CO 3 , Where bis(dibenzylideneacetone)palladium is 2% of 1-chloroanthraquinone, and the molar ratio of bis(dibenzylideneacetone)palladium to large hindered phosphinoferrocene is 1:1.2, Cs 2 CO 3 The molar ratio to 1-chloroanthraquinone is 1:1. Under catalysis, reflux for 36 hours under nitrogen at 90°C, cool to room temperature, vacuum the solvent toluene to keep the solid, and dissolve the remaining solid in dichloromethane , Washed with water 5 times, separated the organic phase in a separatory funnel, added excess anhydrous magnesium sulfate to dry, filtered to retain the filtrate, concentrated the dichloromethane solvent in vacuo, and th...
Embodiment 2
[0077] A preparation method of aniline anthraquinone transition metal complex, including the following steps:
[0078] (1) Dissolve 1-chloroanthraquinone and 2,6-diisopropylaniline in a molar ratio of 1:1.8 in 1,4-dioxane, and then add the catalytic system, the catalytic system is bis( Dibenzylideneacetone)palladium, large hindered phosphinoferrocene and Cs 2 CO 3 , Wherein the bis(dibenzylideneacetone)palladium is 4% of 1-chloroanthraquinone, the molar ratio of bis(dibenzylideneacetone)palladium to the large hindered phosphine ferrocene is 1:1.5, Cs 2 CO 3 The molar ratio with 1-chloroanthraquinone is 1.5:1. Under catalysis, reflux under nitrogen at 110℃ for 36h, cool to room temperature, vacuum the solvent 1,4-dioxane to keep the solid, and remove the remaining The solid was dissolved in dichloromethane, washed with water for 5 times, the organic phase was separated by a separatory funnel, dried by adding excess anhydrous magnesium sulfate, filtered to retain the filtrate, the d...
Embodiment 3
[0086] A preparation method of aniline anthraquinone transition metal complex, including the following steps:
[0087] (1) Dissolve 1-chloroanthraquinone and 2,6-dimethoxyaniline with a molar ratio of 1:0.8 in 1,4-dioxane, and then add the catalytic system, the catalytic system is bis( The molar ratio of dibenzylidene acetone) palladium, large hindered phosphino ferrocene and phosphino ferrocene is 1:2, Cs 2 CO 3 The molar ratio to 1-chloroanthraquinone is 2:1. Under catalysis, reflux for 12 hours under nitrogen at 130°C, cool to room temperature, vacuum the solvent 1,4-dioxane to dry and retain the solid. The solid was dissolved in dichloromethane and washed with water for 5 times. The organic phase was separated by a separatory funnel, dried by adding excess anhydrous magnesium sulfate, filtered to retain the filtrate, the dichloromethane solvent was concentrated in vacuo, and then n-hexane was added to precipitate a large amount of red solid. The solid was filtered under reduc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| Branching factor | aaaaa | aaaaa |
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com