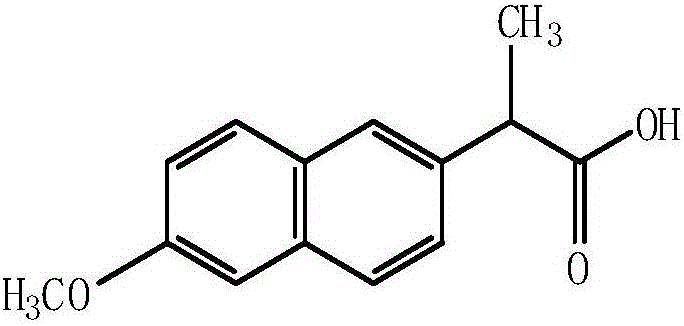
Preparation method of medicine composition containing naproxen
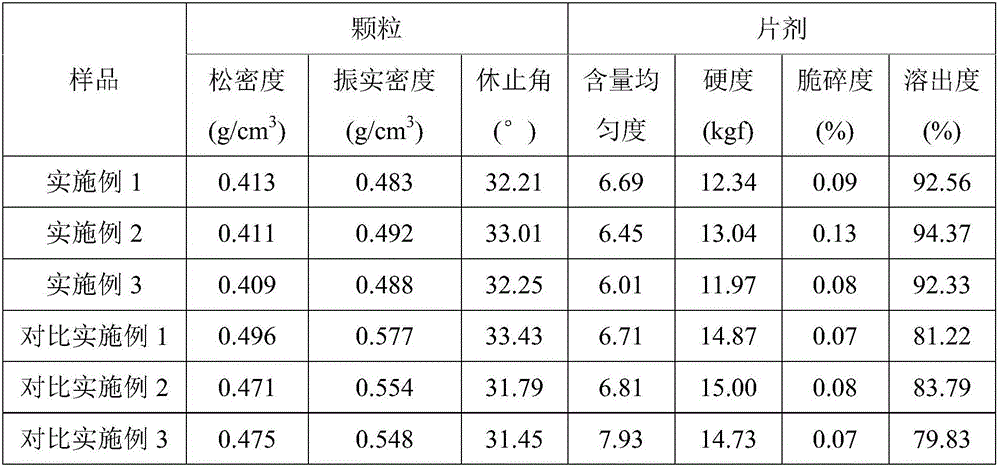
A technology of naproxen and its composition, which is applied in the field of fluidized bed production of naproxen direct-pressed granules, can solve the problems of slow disintegration and dissolution of naproxen tablets, improve the dissolution rate of drugs, and solve the problem of slow disintegration and dissolution , good effect of compression formability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Naproxen 420g
[0034] Lactose monohydrate 60g
[0035] Microcrystalline Cellulose 60g
[0036] Crospovidone 24g
[0037] Hypromellose 30g
[0039] The preparation method is as follows:
[0040] 1) Pass the naproxen bulk drug through a 120-mesh sieve; pass the remaining auxiliary materials through an 80-mesh sieve;
[0041] 2) Preparation of the adhesive: prepare a 6% solution according to the volume ratio of the mass of the adhesive to the purified water of 6:100, and place it on the constant temperature heating stirrer 6 to continue stirring;
[0042] 3) weigh the processed naproxen, lactose, and microcrystalline cellulose according to the prescription quantity, and weigh and mix crospovidone according to 50% of the prescription quantity;
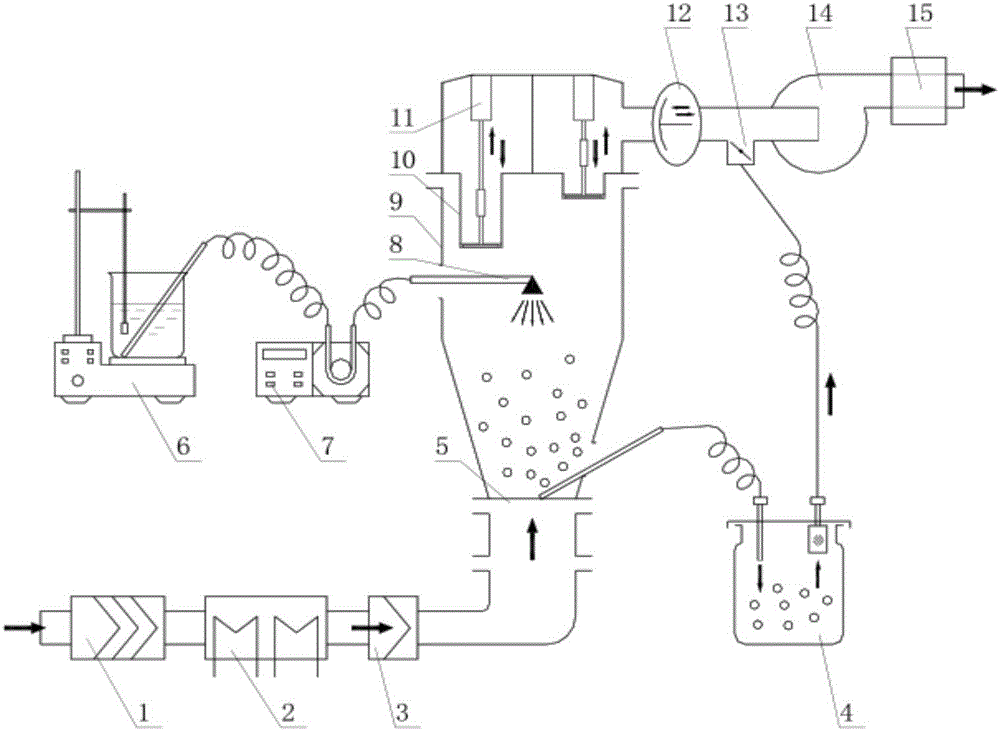
[0043] 4) Start the fluidized bed program, lock the air valve 12 in the sealing chamber, start the fan, and suck the mixed raw and auxiliary materials into the fluidized bed through the vacuum s...
Embodiment 2
[0051] Naproxen 480g
[0052] Starch 48g
[0053] Croscarmellose Sodium 15g
[0054] Sodium carboxymethyl starch 15g
[0055] Hydroxypropyl Cellulose 36g
[0057] Operation process with reference to embodiment 1, difference is as follows:
[0058] 1) Pass the naproxen bulk drug through a 120-mesh sieve; pass the remaining auxiliary materials through an 80-mesh sieve;
[0059]2) Preparation of adhesive: prepare a 5% solution according to the volume ratio of the mass of adhesive to purified water of 5:100;
[0060] 3) The processed naproxen, starch, sodium carboxymethyl starch are weighed and mixed according to the prescription quantity;
[0061] 4) Inhale the mixed raw and auxiliary materials into the fluidized bed, spray the adhesive into the granulation by top spraying, the parameters are as follows:
[0062] Air volume (m 3 / h): 70-80
[0063] Atomization pressure (kg / cm 2 ): 1.3-1.5
[0064] Inlet air temperature (℃): 75-80
[0065...
Embodiment 3
[0069] Naproxen 540g
[0070] Croscarmellose Sodium 12g
[0071] Low-substituted hydroxypropyl cellulose 12g
[0072] Povidone K30 31.2g
[0073] Colloidal silicon dioxide 2g
[0075] Operation process with reference to embodiment 1, difference is as follows:
[0076] 1) Pass the naproxen bulk drug through a 120-mesh sieve; pass the remaining auxiliary materials through an 80-mesh sieve;
[0077] 2) Preparation of the adhesive: prepare an 8% solution according to the volume ratio of the mass of the adhesive to the purified water of 8:100;
[0078] 3) weigh and mix the processed naproxen and low-substituted hydroxypropyl cellulose according to the prescription quantity;
[0079] 4) Inhale the mixed raw and auxiliary materials into the fluidized bed, spray the adhesive into the granulation by top spraying, the parameters are as follows:
[0080] Air volume (m 3 / h): 70-80
[0081] Atomization pressure (kg / cm 2 ): 1.3-1.5
[0082] Inlet air te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com