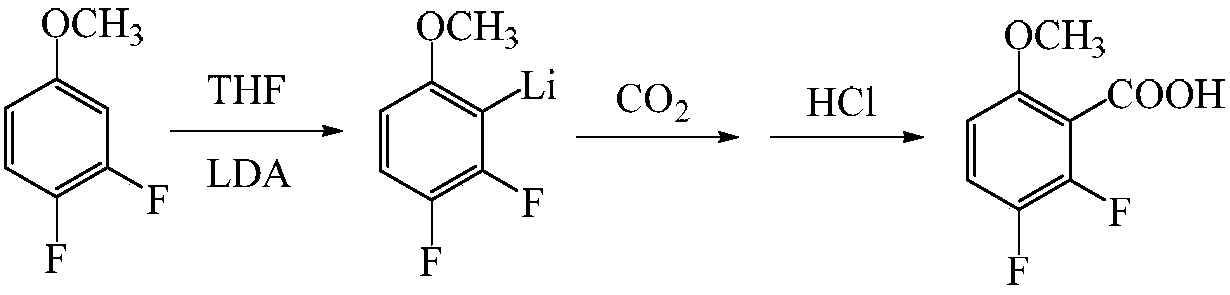
A kind of preparation method of 2,3-difluoro-6-methoxybenzoic acid
A technology of methoxybenzoic acid and difluoroanisole, which is applied in the field of compound preparation, can solve the problems of high cost and impact on large-scale industrial production, and achieve the effects of simplifying the production process, reducing production costs and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Add 4000mL tetrahydrofuran and 463g diisopropylamine to the reaction flask, cool the system to -20°C with liquid nitrogen, stir under nitrogen protection, add dropwise 1800mL n-butyllithium (concentration is 2.5mol / L, the same below ), the temperature is controlled not to exceed -10°C, and after the dropwise addition is completed, a tetrahydrofuran solution of lithium diisopropylamide is obtained.
[0032] (2) Add 600g of 3,4-difluoroanisole and 6000mL of tetrahydrofuran to prepare a tetrahydrofuran solution of 3,4-difluoroanisole, lower the temperature to -70°C, and add lithium diisopropylamide to the tetrahydrofuran solution The tetrahydrofuran solution of the prepared 3,4-difluoroanisole was added dropwise, the temperature was controlled not to exceed -70°C, and the reaction was kept for 60 minutes after the dropwise addition was completed.
[0033] (3) Feed CO into the reaction solution at 2.3L / min 2 Gas, keep the temperature at -75~-70℃, pass CO 2 After 1 h o...
Embodiment 2
[0037] (1) Add 4000mL tetrahydrofuran and 450g diisopropylamine into the reaction flask, cool the system to -25°C with liquid nitrogen, stir under nitrogen protection, add 1700mL n-butyllithium dropwise, control the temperature not to exceed -10°C, drop After the addition was complete, a solution of lithium diisopropylamide in tetrahydrofuran was obtained.
[0038] (2) Add 580g of 3,4-difluoroanisole and 6500mL of tetrahydrofuran to prepare a tetrahydrofuran solution of 3,4-difluoroanisole, lower the temperature to -70°C, and add lithium diisopropylamide to the tetrahydrofuran solution The tetrahydrofuran solution of 3,4-difluoroanisole prepared was added dropwise, the temperature was controlled not to exceed -70°C, and the reaction was kept for 50 minutes after the dropwise addition was completed.
[0039] (3) Feed CO into the reaction solution at 2.2L / min 2 Gas, keep the temperature at -75~-70℃, pass CO 2 After 1 h of gas, the reaction was stirred for 30 min.
[0040] (4)...
Embodiment 3
[0043] (1) Add 5000mL tetrahydrofuran and 500g diisopropylamine into the reaction flask, cool the system to -20°C with liquid nitrogen, stir under nitrogen protection, add 2000mL n-butyllithium dropwise, control the temperature not to exceed -10°C, drop After the addition was complete, a solution of lithium diisopropylamide in tetrahydrofuran was obtained.
[0044] (2) Add 650g of 3,4-difluoroanisole and 7000mL of tetrahydrofuran to prepare a tetrahydrofuran solution of 3,4-difluoroanisole, lower the temperature to -70°C, and add lithium diisopropylamide to the tetrahydrofuran solution The tetrahydrofuran solution of the prepared 3,4-difluoroanisole was added dropwise, the temperature was controlled not to exceed -70°C, and the reaction was kept for 70 minutes after the dropwise addition was completed.
[0045] (3) Feed CO into the reaction solution at 2.5L / min 2 Gas, keep the temperature at -75~-70℃, pass CO2 After 1 h of gas, the reaction was stirred for 30 min.
[0046] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com