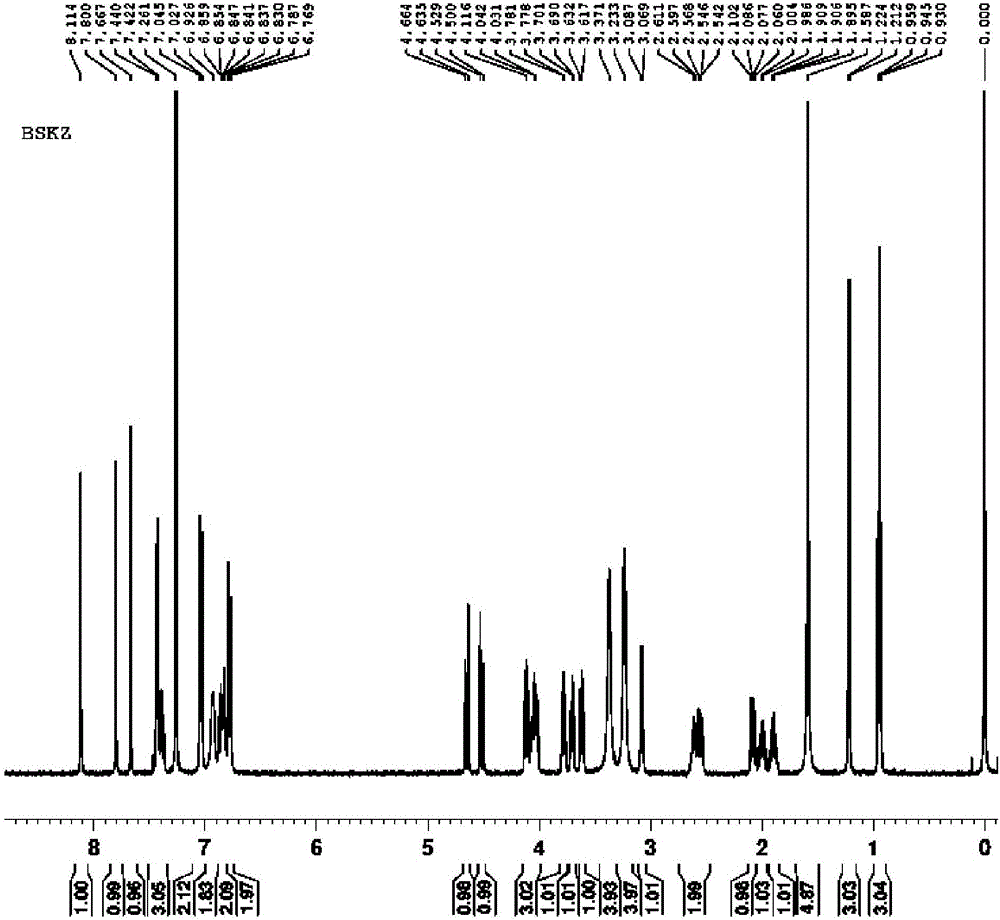
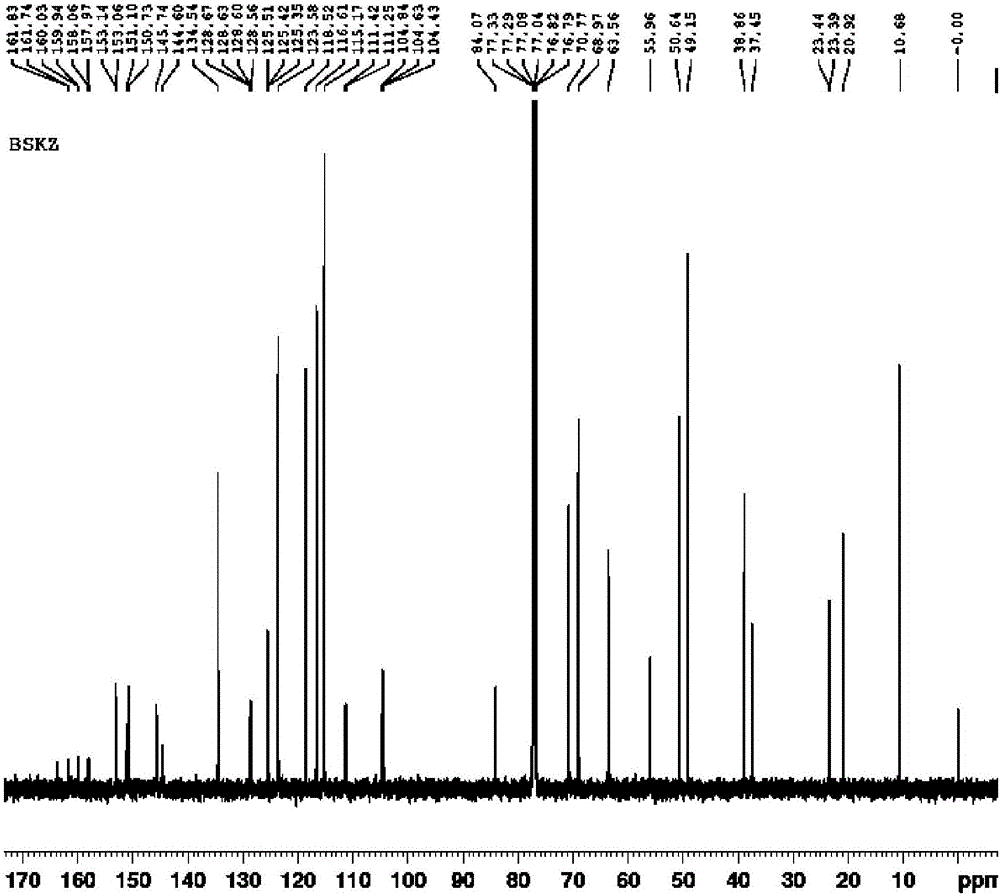
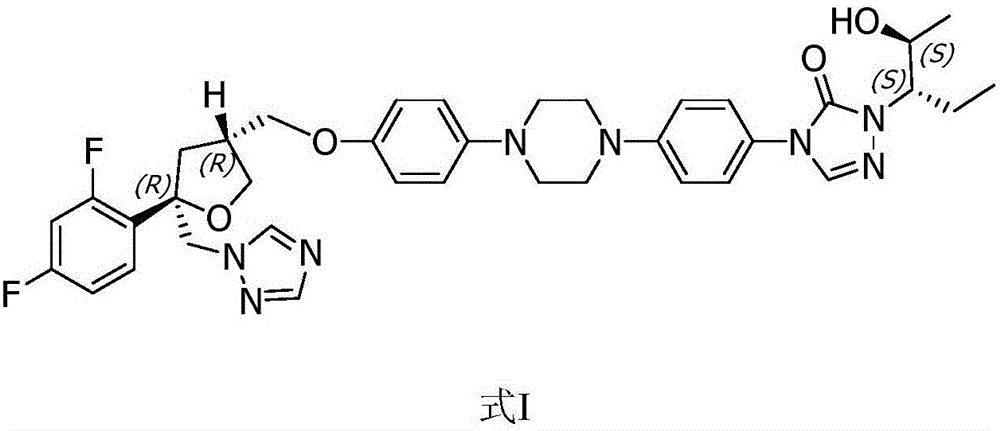
Posaconazole synthesis method
A technique for the synthesis of posaconazole, which is applied in the field of drug synthesis, can solve the problems of cumbersome post-processing, easy oxidation, and low yield, and achieve the effects of simple post-processing, shortened reaction time, and reduced pressure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] For illustrating the present invention posaconazole and its synthetic method
[0042] (1) Reactor structure with built-in agitator:
[0043] The reactor has a height of 120cm and a diameter of 100L enamel reactor of 50cm. There is a stirrer and a set of baffle plates, the stirrer is standard, including a stirring shaft arranged along the axis of the reactor and two sets of stirring blades uniformly distributed along the axis of the stirring shaft Groups, each group of stirring blade groups are arranged in parallel, and each group of stirring blade groups includes two stirring blades uniformly distributed along the circumference of the stirring shaft, and the length of each stirring blade along the radial direction of the reactor is 8cm; The plate group includes a height of 60 cm, a width of 5 cm, and a thickness of 4 baffles of 1 cm. The length direction of each baffle is arranged along the axial direction parallel to the stirrer (one side is fixed on the bottom wall o...
Embodiment 2
[0047] For illustrating the present invention posaconazole and its synthetic method
[0048] (1) Built-in reactor structure of agitator: same as embodiment 1.
[0049] (2) Synthesis of posaconazole:
[0050] Add in the enamel reactor of aforementioned 100L, add 60L methyl alcohol, compound shown in 4kg formula II, 6L concentration is the sulfuric acid of 98wt%, 10% palladium carbon catalyst (water content 50%) of 400g, seal reactor, open stirrer ( Stirring speed is 400rpm). First replace the air in the kettle with nitrogen for 3 times, then replace it with hydrogen for 3 times, keep the pressure of hydrogen at 0.1 MPa (gauge pressure), raise the temperature to 55°C, monitor the reaction by HPLC, and the reaction is completed after 2 hours. Cool down, replace with nitrogen for 3 times, and filter to obtain 10% palladium carbon catalyst. Pour the filtrate into another 200L enamel reaction kettle, add ice cubes, cool down to 0-5°C, add 2mol / L NaOH solution dropwise to the filt...
Embodiment 3
[0052] For illustrating the present invention posaconazole and its synthetic method
[0053] (1) built-in reactor structure with stirrer: same as embodiment 1;
[0054] (2) Synthesis of posaconazole:
[0055] Add in the enamel reactor of aforementioned 100L, add 60L methyl alcohol, the compound shown in 4kg formula II, 4L concentration is the hydrochloric acid of 36.5wt%, the 10% palladium carbon catalyst (water content 50%) of 200g, seal reactor, open stirrer (Stirring speed is 450rpm). First replace the air in the kettle with nitrogen for 3 times, then replace it with hydrogen for 3 times, keep the pressure of hydrogen at 0.12MPa, raise the temperature to 50°C, monitor the reaction by HPLC, and the reaction is completed in 3 hours. Cool down, replace with nitrogen for 3 times, and filter to obtain 10% palladium-carbon catalyst (standby). Pour the filtrate into another 200L enamel reaction kettle, add ice cubes, cool down to 0-5°C, add 2mol / L Na to the filtrate dropwise 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com