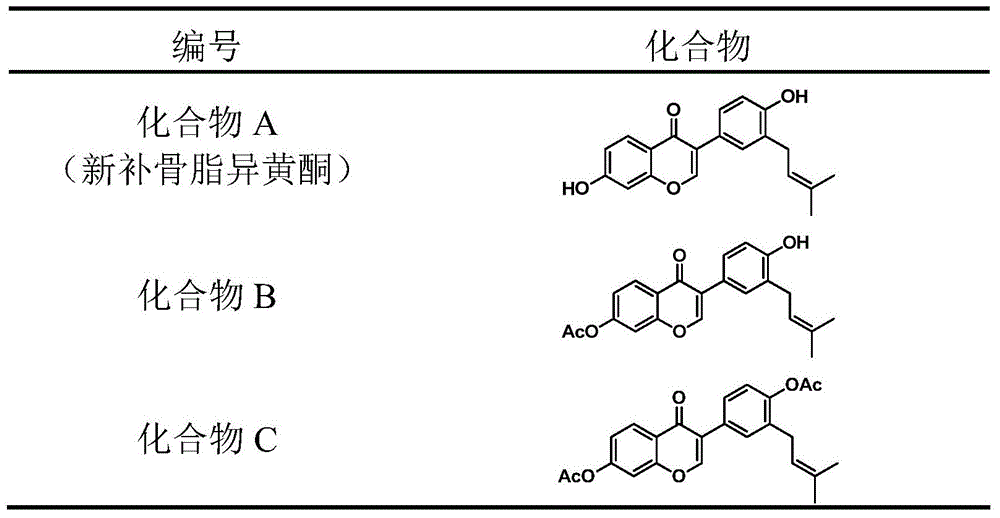
A class of glucuronosyltransferase UGT1A1 inducers and applications thereof
A technology of glucuronic acid and inducer, which is applied in the field of medicine, can solve serious adverse reactions and other problems, and achieve the effects of avoiding the first-pass effect, strong induction ability, and eliminating hyperbilirubinemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of 4',7-dihydroxy-3'-prenyl isoflavone diacetyl derivatives
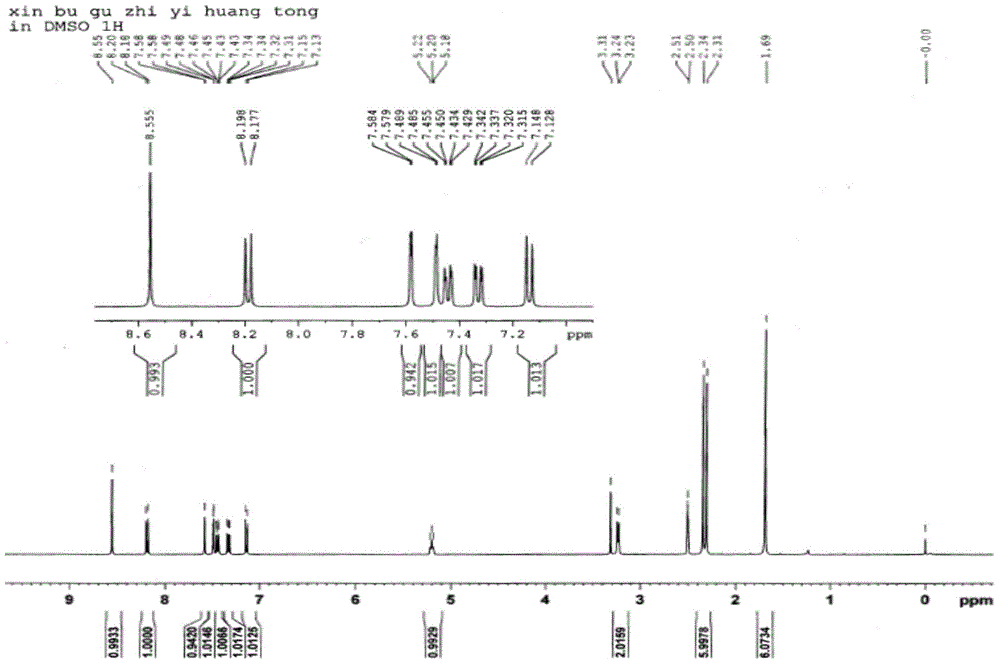
[0028] Weigh 4',7-dihydroxy-3'-prenyl isoflavone ( figure 1 , Compound A) 200 mg was dissolved in 10 mL of dichloromethane, 0.5 mL of triethylamine was added, the reaction solution was cooled to 0° C., and 100 μL of acetyl chloride was slowly added dropwise. After reacting for 1 hour, TLC detected that the reaction was complete, slowly added 20 mL of water, extracted twice with 30 mL of dichloromethane, combined the organic phases, washed with water, dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness under reduced pressure to obtain a crude product. The crude product 4mL methanol was heated to reflux for 30min, cooled, filtered, and the filter cake was collected and vacuum-dried to obtain 4',7-dihydroxy-3'-prenyl isoflavone diacetyl derivative ( figure 1 , Compound C) 230mg, white solid, yield 91%. Wherein the NMR spectrum of 4,7-dihydroxy-3'-prenyl isoflav...
Embodiment 2
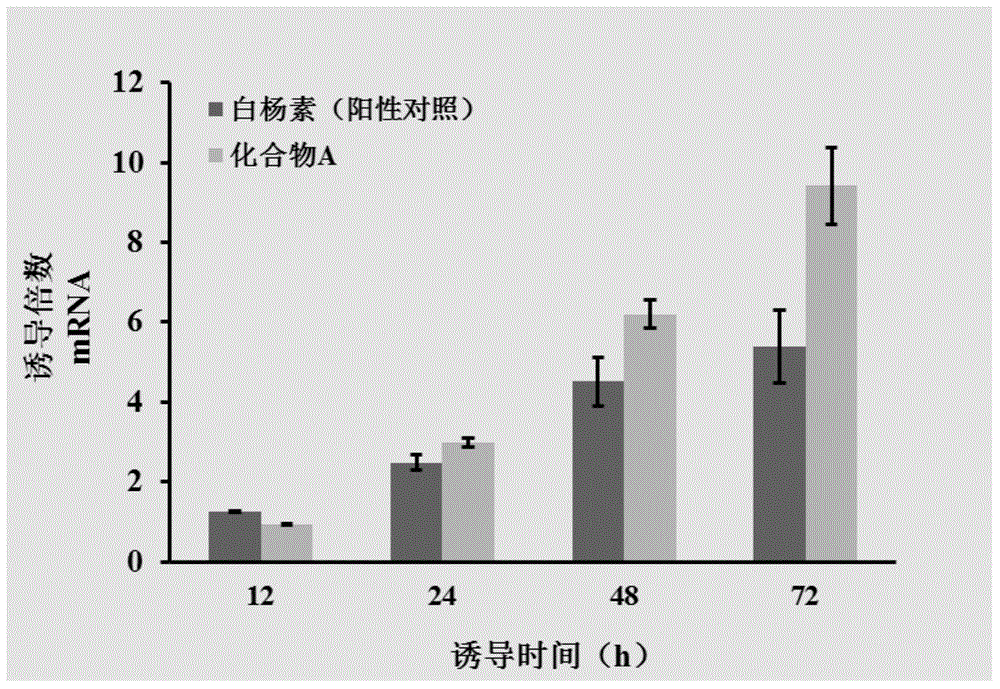
[0032] The ability of this class of drugs to induce the expression of UGT1A1 enzyme mRNA
[0033]Inoculate Caco-2 in a 24-well plate, and after incubation for 24 hours, replace the medium with fresh drug-containing medium containing 4',7-dihydroxy-3'-prenyl isoflavone, and the final concentration of the compound is 25 μM. The fresh medium containing the drug was replaced every day, and the cells were collected with RNA extraction reagent after culturing for 12, 24, 48, and 72 hours, respectively. Total mRNA was extracted according to the procedure attached to the RNA extraction reagent, and the mRNA was reverse-transcribed into cDNA using a reverse transcription kit. Subsequently, the mRNA expression level of UGT1A1 enzyme in the cells was measured with a real-time PCR instrument. By measuring the ability of new psoralen isoflavones to induce the expression of UGT1A1 enzyme over time ( image 3 ) It can be seen that the induction ability of 4',7-dihydroxy-3'-prenyl isoflavo...
Embodiment 3
[0035] Fluorescent probe method to determine the ability of this kind of drugs to induce UGT1A1 enzyme activity
[0036] Caco-2 cells were seeded at 75cm 2 When culturing to 70% confluency, add 25 μM 4',7-dihydroxy-3'-prenyl isoflavone and its acetylated derivatives, and replace the drug-containing fresh medium every day during the culture period until incubation for 3 sky. After incubation, the cells were collected by trypsinization and washed three times with ice-cold PBS. Add PBS containing 1% cocktail protease inhibitor to the cell pellet, place it on ice for sonication, then centrifuge at 9000 g for 20 min, and collect the supernatant as cell S9 component, which is used to determine the activity of UGT1A1 enzyme. The assay procedure of UGT1A1 enzyme activity is as follows:
[0037] (1) 200 microliters of in vitro metabolic reaction system contains 50mM Tris-Hcl buffer (PH=7.4), the protein concentration of cell S9 is 1mg / ml, Mgcl 2 5mM, UGT1A1 probe substrate concentr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Protein concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com