Surface coating composition of implantable medical apparatus, medical apparatus and manufacturing method of medical apparatus
A technology for implanting medical devices and surface coatings, applied in the field of medical devices, can solve the problems of unsuitable degradable vascular stents, etc., and achieve the effects of excellent drug controlled release performance, controllable process, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
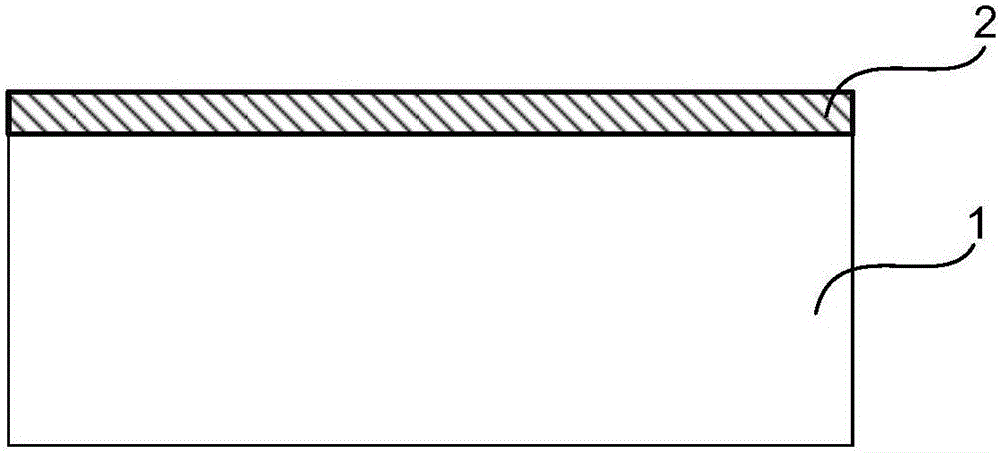
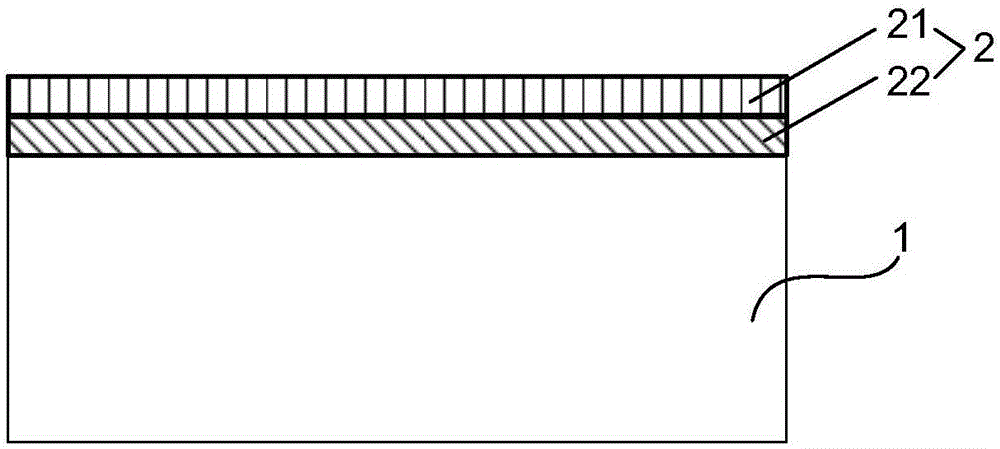
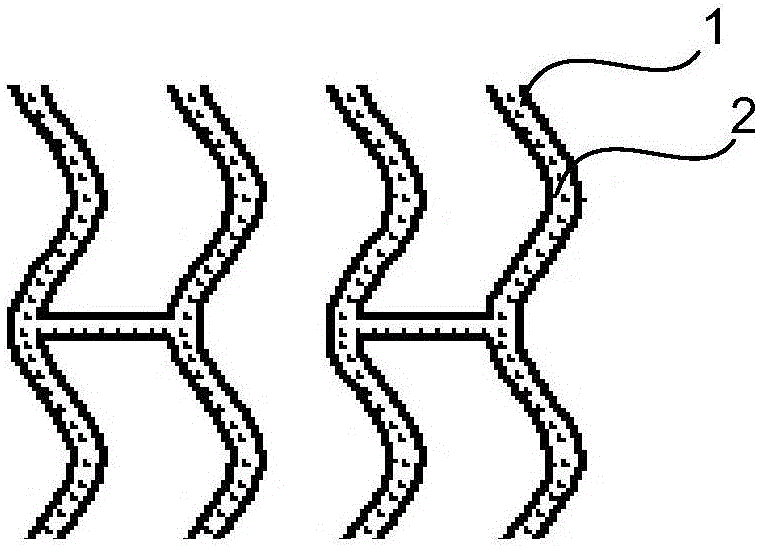
[0068] Such as figure 1 As shown, the drug-loaded coating 2 is located on the outer surface of the degradable stent skeleton 1 (i.e. the outer surface of the cylindrical stent), and the drug-loaded coating 2 is a mixture composed of therapeutic drugs and degradable carriers, so The above-mentioned therapeutic drugs can be antithrombotic drugs: heparin, hirudin, prostacyclin, abciximab, etc.; anti-inflammatory drugs: dexamethasone (DXM), methylprednisolone, bisphosphonate liposomes, etc. ; Anti-VSMC proliferation drugs: rapamycin (RAPM), paclitaxel, angiopep, mycophenolic acid, tacrolimus, everolimus, cyclosporine A, methyl-RAPM, etc.; anti-VSMC migration drugs: Batimastat, etc.; drugs for promoting endothelial healing: 17β estradiol, vascular endothelial growth factor, etc.; other drugs: one or more of tetratoxazine and its combination with traditional Chinese medicines astragalus, ginseng, emodin, etc. The degradable carrier can be one of polylactic acid (PLLA and PDLLA) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com