A 5-hydroxytryptamine receptor agonist, a preparing method thereof and uses of the receptor agonist
A serotonin and solid technology, applied in the field of medicinal chemistry, can solve the problems of poor solubility and low stability, and achieve the effects of easy operation, high stability, superior formability and
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh 20g of tandospirone citrate, add 140mL of water, heat up to 70°C, wait for complete dissolution, cool down to 40±5°C, add 100mL of acetone and 100mL of diethyl ether under stirring, continue to cool down to room temperature and stir for 1 hour, 5 Stir at ±5°C for 1 hour, wait for the solid to precipitate, filter, and vacuum dry at 50°C for 2 hours to obtain 19.6 g of tandospirone citrate 4 / 5 hydrate with a yield of 95.6% and a purity of 99.93%.
[0038] Mass spectrum showed its ESI m / z: 383.
[0039] Get product of the present invention to carry out elemental analysis, the results are shown in Table 1.
[0040] Table 1. Elemental analysis results
[0041] element N% C% H% C 21 h 29 N 5 o 2 · C 6 h 8 o 7 · 4 / 5H 2 O theoretical value
11.87 54.96 6.59 The measured value of the product of the present invention 11.88 54.94 6.58
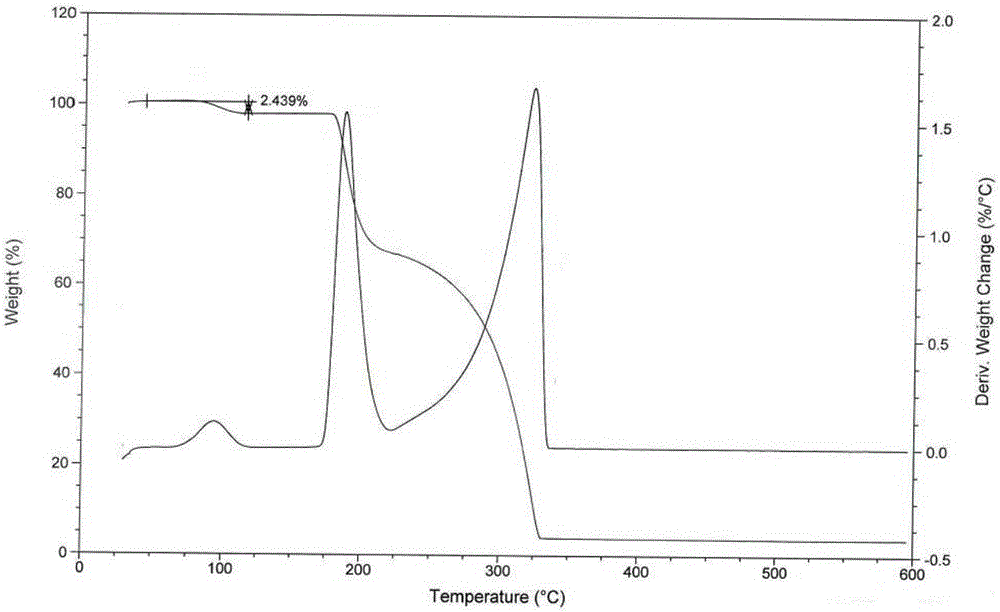
[0042] Determination of loss on drying: the product of the present invention was dri...
Embodiment 2
[0049] Weigh 20g of tandospirone citrate, add 300mL of water, heat up to 50°C, wait for complete dissolution, cool down to 40±5°C, add 150mL of acetone and 250mL of diethyl ether under stirring, continue to cool down to room temperature and stir for 3 hours, 5 Stir at ±5°C for 1 hour, wait for the solid to precipitate, filter, and vacuum dry at 35°C for 8 hours to obtain 19.8 g of tandospirone citrate 4 / 5 hydrate with a yield of 96.6% and a purity of 99.90%.
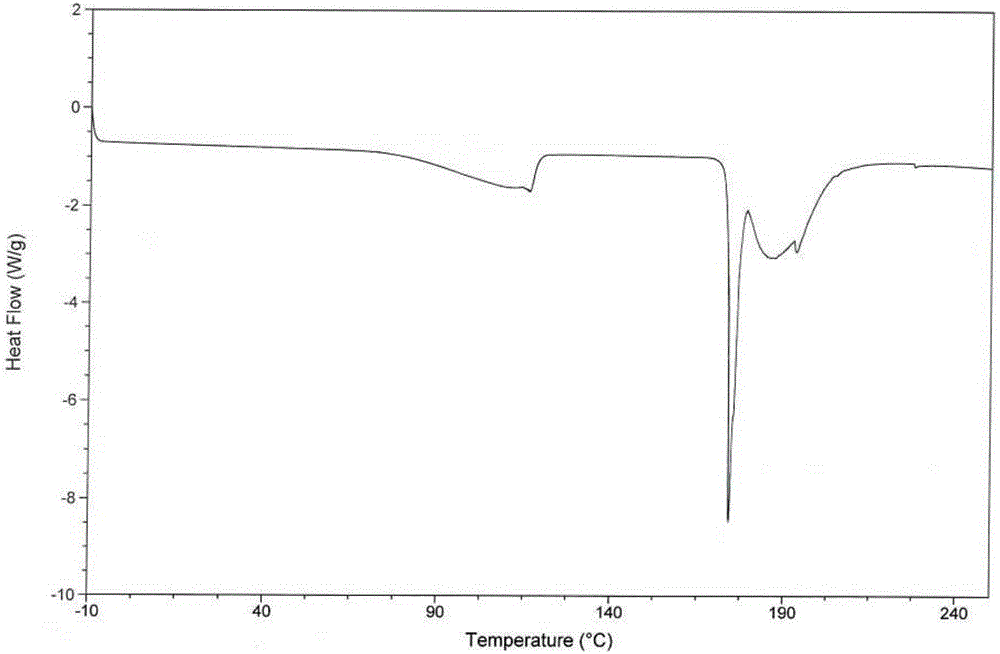
[0050] The test results of elemental analysis, loss on drying, moisture determination and thermal analysis of the obtained product show that what the present invention obtains is tandospirone citrate 4 / 5 hydrate.
Embodiment 3
[0052] Weigh 20g of tandospirone citrate, add 100mL of water, heat up to 90°C, wait for complete dissolution, cool down to 40±5°C, add 40mL of acetone and 60mL of ether under stirring, continue to cool down to room temperature and stir for 15 minutes, 5 Stir at ±5°C for 4 hours, wait for solid precipitation, filter, and vacuum dry at 15°C for 10 hours to obtain 19.5 g of tandospirone citrate 4 / 5 hydrate with a yield of 95.1% and a purity of 99.93%.
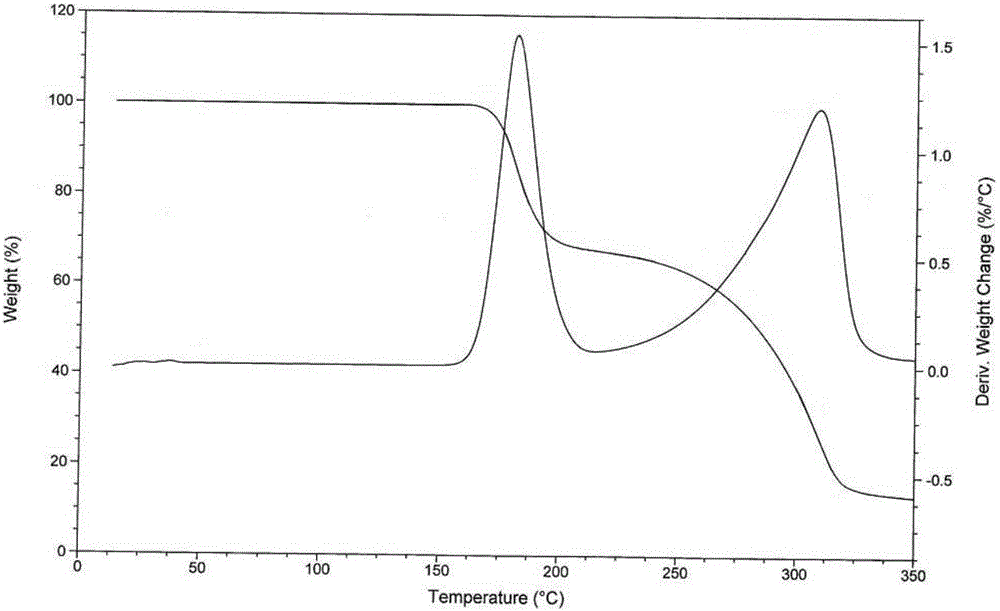
[0053] The test results of elemental analysis, loss on drying, moisture determination and thermal analysis of the obtained product show that what the present invention obtains is tandospirone citrate 4 / 5 hydrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com