Oral porcine circovirus II-like particle vaccine, and preparation method and application thereof
A porcine circovirus and defect-type technology, applied in biochemical equipment and methods, vaccines, viruses, etc., can solve problems such as low production efficiency, inability to take oral administration, and large side effects, and achieve low production costs, good immune effects, and safety sex high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
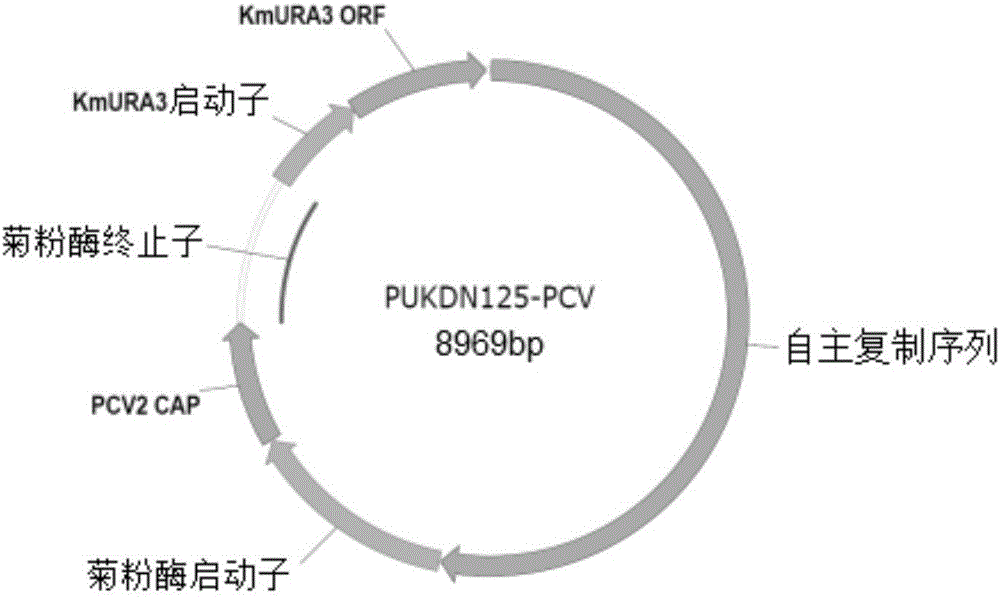
[0064] Embodiment 1, the construction of PCV capsid protein recombinant expression vector pUKD-N125 / Cap
[0065] In this embodiment, the gene encoding the amino acid sequence of porcine circovirus capsid protein (hereinafter also referred to as "porcine circovirus capsid Cap protein", or "Cap protein") has the sequence of SEQ ID No.3. According to the codon preference of Kluyveromyces marx, the codons of the Cap gene sequence were optimized, and the optimized Cap gene sequence was artificially synthesized.
[0066] The amino acid sequence of the encoded Cap protein can be as shown in SEQ ID No.1, or the phenylalanine at position 226 in SEQ ID No.1 is replaced by leucine (such as SEQ ID No.2).
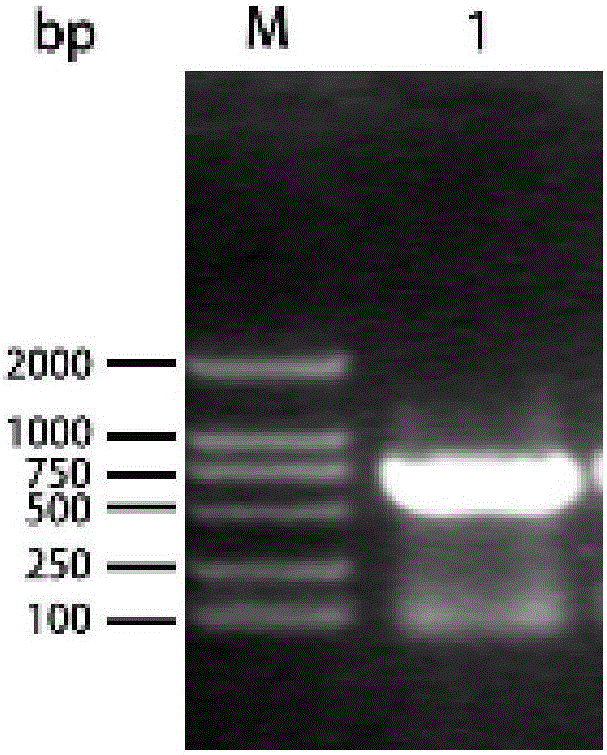
[0067] Using the method of PCR amplification, the Cap gene was amplified with primers PCVN125-F (5'-TTTTTTTTGTTAGATCCGCGGATGACATATCCAAGGAGGCGTTTC-3') and PCVN125-R (5'-AGCTTGCGGCCTTAACTAGTTCA CTTAGGGTTAAGTGGAGGGTCCTTAAG-3'). After 1% agarose gel electrophoresis, a fragment of about 700...
Embodiment 2
[0074] Example 2, Construction of PCV Capsid Protein Cap Protein in Kluyveromyces marx Genetic Engineering Bacteria Fim-1ura3Δ-pUKD-N125 / Cap and Cap Expression Analysis
[0075] A uracil auxotrophic strain Fim-1ura3Δ of Kluyveromyces marxense is provided, and the preparation method of the strain can refer to the construction method of the Fim-1 (ura3Δ) strain disclosed in Example 1 of Chinese patent publication CN105112313A.
[0076] Fim-1ura3Δ was inoculated in a glass test tube containing 3 mL of YEPD medium, and cultured overnight on a shaker at 30°C until the OD600 was 12-15. The cells were collected and washed with LiAc-TE solution (100 mM LiAc, 10 mM Tris-HCl, 1 mM EDTA).
[0077] Add carrier DNA, recombinant carrier pUKD-N125 / Cap, PEG solution (40% PEG4000, 100mM LiAc, 10mM Tris-HCl pH 7.5, 1mM EDTA) and a final concentration of 10mM DTT to the cells in sequence, and mix thoroughly. Water bath at 30°C for 15 minutes, water bath at 47°C for 15 minutes, spin off at 8000 ...
Embodiment 3
[0079] Example 3, Kluyveromyces marx genetically engineered bacteria Fim-1ura3Δ-pUKD-N125 / Cap expressing Cap protein and assembly of virus-like particles
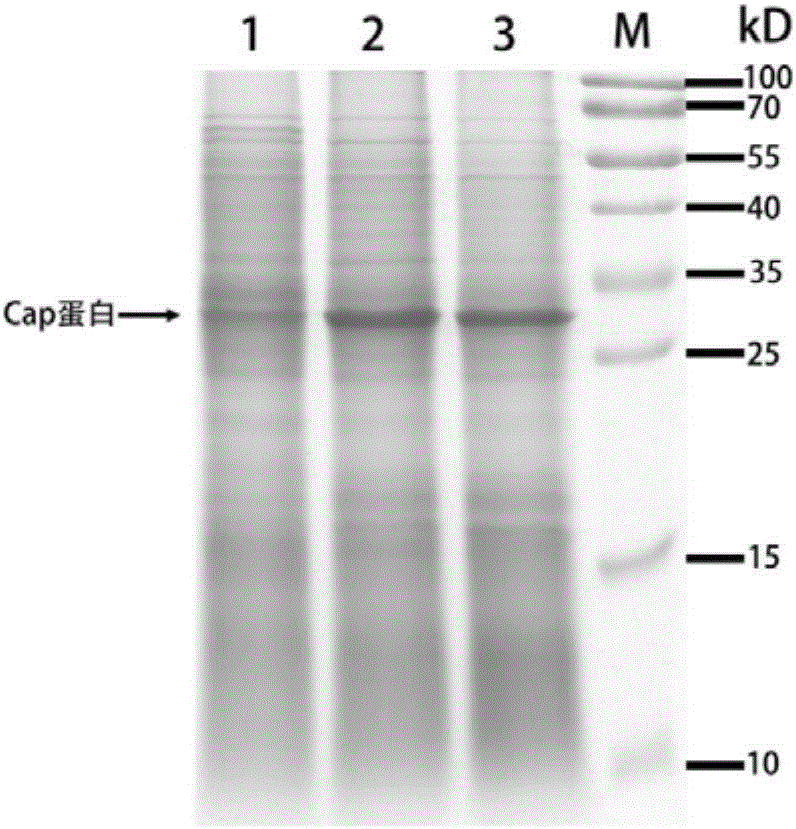
[0080] The positive clones verified by PCR were inoculated in a Erlenmeyer shaker flask containing 50 ml of YP medium (1% Yeast Extract, 2% glucose), cultured at 30°C and 220rpm for 96h, and centrifuged to collect cells, and homogenized by high pressure The method was used to disrupt the cells, and the cell lysate was subjected to polyacrylamide gel electrophoresis (SDS-PAG) (polyacrylamide gelelectrophoresis, PAGE) and Western Blot with PCV antibody to detect the expression of porcine circovirus (PCV) Cap protein in Kluyveromyces.
[0081]Compared with the control bacteria Fim-1ura3Δ (lane 1), there is an extra protein of 35-40kD in the whole cell lysate and supernatant (lanes 2 and 3) of the genetically engineered bacteria Fim-1ura3Δ-pUKD-N125 / Cap strips (such as image 3 ), this protein band is the Cap protein of porcin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com