Primer group and kit for detecting diarrhea-causing parasites through multi-PCR technology
A parasite and primer set technology, applied in the biological field, can solve the problems of high missed detection rate, difficulty in comprehensive screening of diarrhea-causing parasite detection technology, etc., and achieve improved sensitivity, high specificity, high conservation and specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
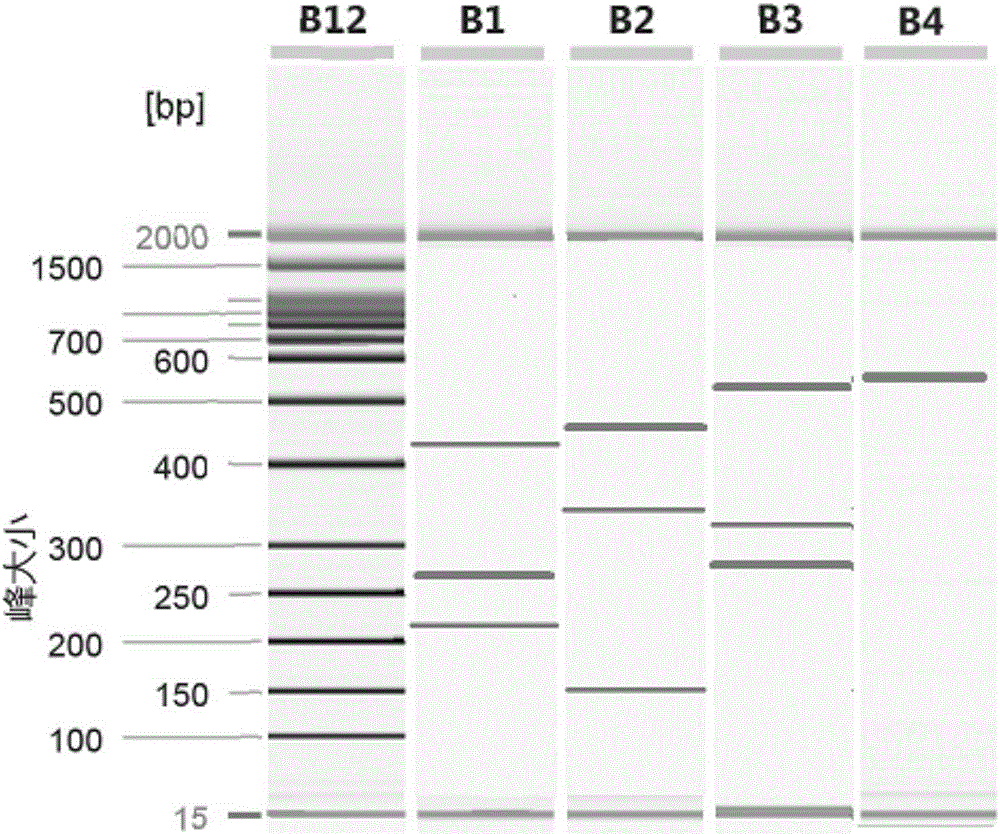
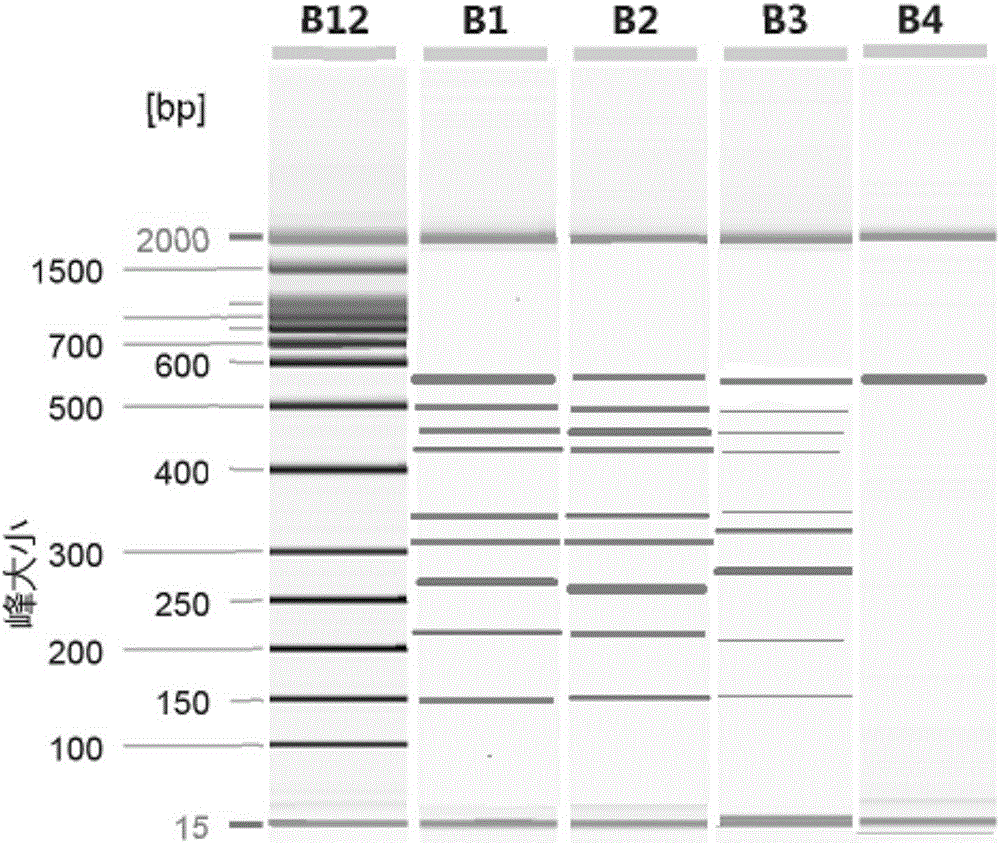
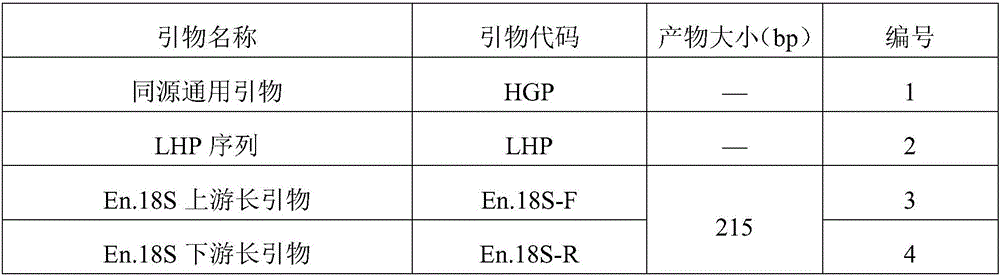
[0087] 1. Primer synthesis: the primers of SEQ ID NO.1 and 3-20 were synthesized. Taking the amount of substance as the unit, take 1 part of each of the primers of SEQ ID NO.3-20 and mix with 4 parts of the primer of SEQ ID NO.1 to form the primer set of this embodiment. See Table 3 for a summary of primers.
[0088] Table 3 Summary of Multiplex PCR Primer Sequences
[0089]
[0090]
[0091] 2. Specificity verification:
[0092] Select 12 unrelated pathogens as simulated interference samples: Salmonella (purchased from China Medical Culture Collection Center, No. 50001), Shigella (purchased from China Medical Culture Collection Center, No. 51054), Vibrio parahaemolyticus (purchased from China Medical Culture Collection Center, No. 21617), Campylobacter jejuni (purchased from China Medical Culture Collection Center, 186089), Staphylococcus aureus (purchased from China Medical Culture Collection Center, No. 26003), wax Bacillus-like bacteria (purchased from China Medic...
Embodiment 2
[0101] 1. Construction of multiplex PCR detection kit
[0102] The kit consists of 2× reaction system buffer, DNA polymerase, 10× primer mixture, positive control, and ultrapure water. The specific components are as follows: 2×PCR Buffer (Tris-HCl 40Mm (pH 8.3), KCl 100mM, tween-200.08%, 0.0006ng / μL PET28a, 1mM dNTP, 8mM MgCl 2 ); 25× DNA polymerase (2U / μL); 10× primer mixture (concentration of long primer pair including IAC is 2 μM each, concentration of homologous universal primer SEQ ID NO.1 is 8 μm); positive control (containing 8 mixed templates of parasites, each at 0.2 ng / μL).
[0103] 2. Genome extraction
[0104] Entamoeba histolytica, Giardia, Cryptosporidium, Schistosoma japonicum, Clonorchis sinensis, Strongyloides sternum, Blastocystis hominis and Paragonimus wescheri were selected as the target parasites for the establishment of the detection method, respectively The eight kinds of parasites were collected according to the national standard or industry standar...
Embodiment 3
[0114] The minimum detection limit test of embodiment 3 kit
[0115] Test samples for evaluation: select 8 parasites, sample 1 (template 1) is Entamoeba histolytica, sample 2 (template 2) is Giardia, sample 3 (template 3) is Cryptosporidium, sample 4 (Template 4) is Schistosoma japonicum, Sample 5 (Template 5) is Clonorchis sinensis, Sample 6 (Template 6) is Strongyloides sternum, Sample 7 (Template 7) is Blastocystis hominis, and Sample 8 (Template 8) is Paragonimus westermani, sample 9 (template 9) is a mixed template of the above eight parasites. The bacterial suspensions of the 9 templates were adjusted to 20ng / ul respectively, and the 9 samples were serially diluted into 2ng / ul, 0.2ng / ul, 0.02ng / ul, 0.002ng / ul and 0.0002ng / ul test samples.
[0116] Templates with different dilutions were detected using the kit of the present invention. Carry out operation and result judgment according to embodiment 2, the minimum detection limit test result that kit detects 8 target gen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com