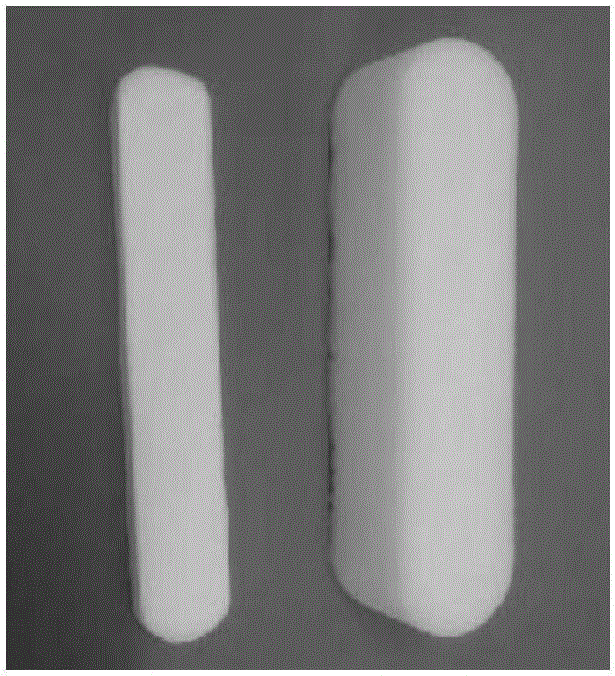
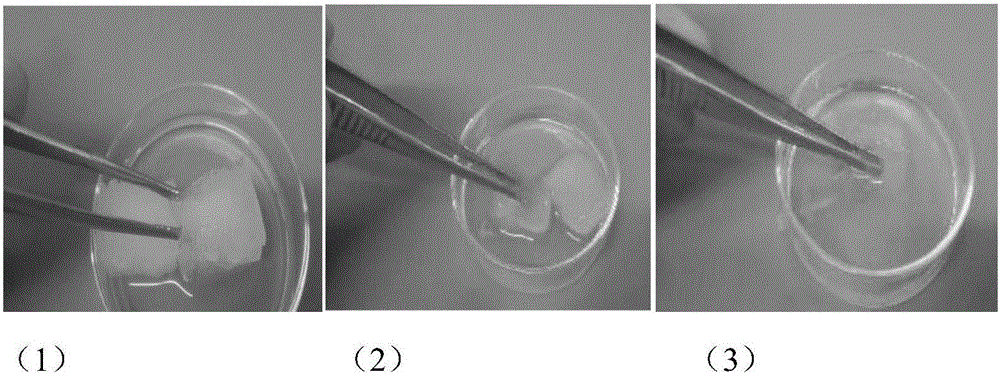
High-expansion degradable nasal cavity filling hemostasis material and preparation method thereof
A hemostatic material and nasal cavity technology, applied in the field of medical biomaterials, can solve the problems that the product cannot be put into the nasal cavity as a whole, cannot fully exert the hemostatic effect of filling, poor biocompatibility, etc., achieves superior physical and chemical properties, and avoids secondary damage to the mucosa Sexual bleeding, strong plasticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
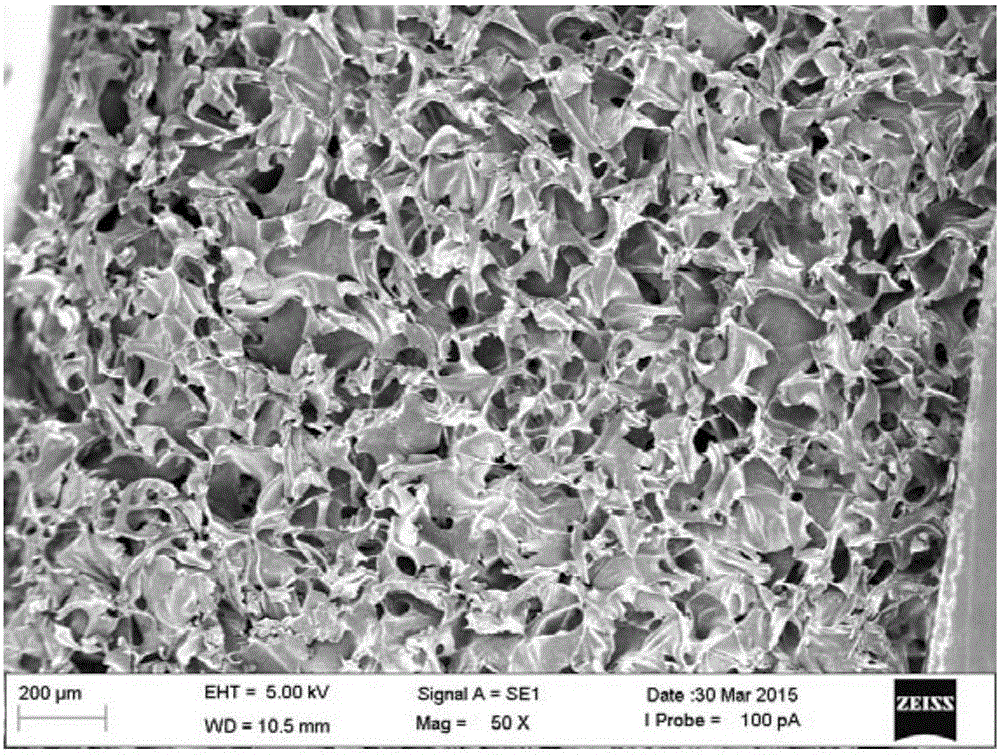
[0043] (1) 0.5 g of medical carboxymethyl chitosan with a degree of substitution of 80% and a viscosity-average molecular weight of 150,000 is placed in 100 g of 50° C. purified water and stirred and dissolved to obtain an aqueous solution of carboxymethyl chitosan, and then a 4-g length Bacterial cellulose with a thickness of 100 μm was added to the above solution for homogenization to obtain a microfiber-suspended carboxymethyl chitosan solution.
[0044] (2) Add 0.1g activator 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide to the amino group and carboxyl group of carboxymethyl chitosan in the suspension to carry out activation treatment, will The feed liquid was poured into the corresponding mold, and left for 1 hour, carboxymethyl chitosan self-crosslinked to form a hydrogel, washed with purified water to remove the activator EDC, and vacuum freeze-dried for 24 hours to obtain an intermediate product, a loose porous sponge.
[0045](3) 100 g of a solution of the degradatio...
Embodiment 2
[0047] (1) 10 g of medical carboxymethyl chitosan with a degree of substitution of 85% and a molecular weight of 200,000 is placed in 100 g of 50° C. purified water and stirred and dissolved to obtain an aqueous solution of carboxymethyl chitosan, and then 0.1 g of the carboxymethyl chitosan with a length of 150 μm Bacterial cellulose was added to the above solution for homogenization treatment to obtain microfiber suspension carboxymethyl chitosan solution.
[0048] (2) Add 5g activator 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide to the amino group and carboxyl group of carboxymethyl chitosan in the suspension to carry out activation treatment, the material The solution was poured into corresponding molds and left for 8 hours. Carboxymethyl chitosan self-crosslinked to form a hydrogel, washed with purified water to remove the activator EDC, and vacuum freeze-dried for 48 hours to obtain an intermediate product, a loose porous sponge.
[0049] (3) Prepare 100 g of a solutio...
Embodiment 3
[0051] (1) 4g of medical carboxymethyl chitosan with a degree of substitution of 90% and a molecular weight of 300,000 is placed in 100g of 50°C purified water and stirred and dissolved to obtain an aqueous solution of carboxymethyl chitosan, and then 2g of bacteria with a length of 350 μm The cellulose is added into the above solution for homogenization treatment to obtain a microfiber suspension carboxymethyl chitosan solution.
[0052] (2) Add 2g activator 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide to the amino group and carboxyl group of carboxymethyl chitosan in the suspension to carry out activation treatment, the material The solution was poured into corresponding molds and left for 8 hours. Carboxymethyl chitosan self-crosslinked to form a hydrogel, washed with purified water to remove the activator EDC, and vacuum freeze-dried for 36 hours to obtain a loose porous sponge as an intermediate product.
[0053] (3) 100 g of a solution of the degradation regulator lyso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com