Preparation method and application of GSH (glutathione) water-soluble fluorescent probe based on rhodamine
A fluorescent probe, water-soluble technology, applied in the field of fluorescent probes, to achieve the effect of good water solubility and rapid response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of fluorescent probes
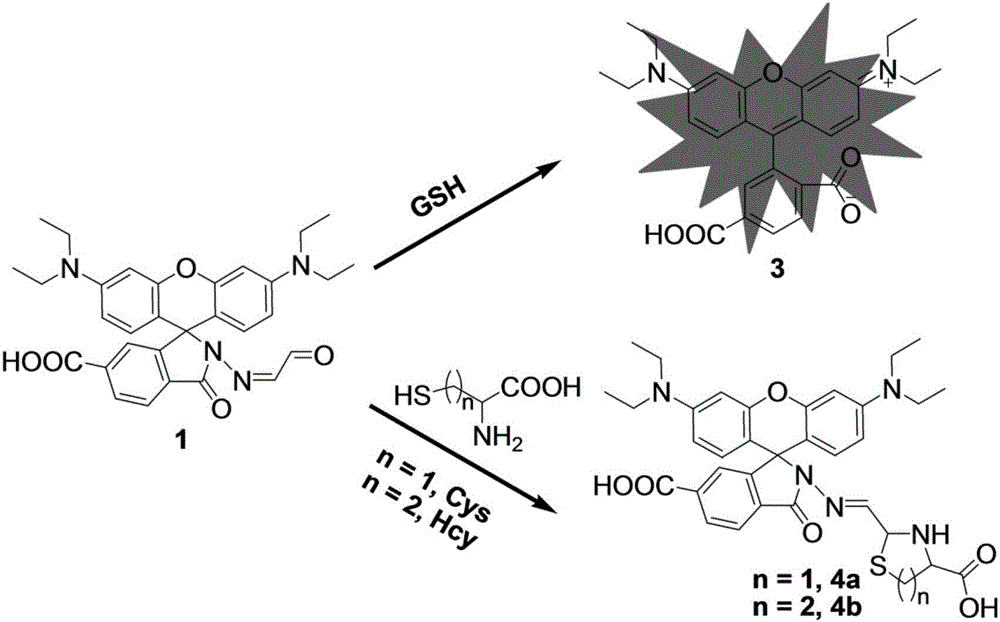
[0030] Synthesis of compound Rh1: 1,2,4-Trimellitic anhydride (1.92 g, 10 mmol) and 3-diethylaminophenol (3.30 g, 20 mmol) were added to a 100 mL round bottom flask containing 50 mL of N,N-dimethylformamide , under the protection of nitrogen, the magnetic stirring was refluxed for 6 hours, and the reaction was stopped; after the reaction mixture was cooled to room temperature, twice distilled water (200mL) was added, and after being fully stirred, it was extracted with dichloromethane (100mL×3), and the organic layers were combined and reduced. The solvent was distilled off under pressure. The crude product was separated by column chromatography with dichloromethane / methanol at 6:1 (volume ratio) to obtain a red solid Rh1 (0.419 g, yield: 8.6%). 1 H NMR (400MHz, CD 3 OD)δ8.19(d,J=8.0Hz,1H),8.08(d,J=8.0Hz,1H),7.80(s,1H),7.28(d,J=8.0Hz,2H),6.98(d ,J=8.0Hz,2H),6.89(s,2H),3.64(d,J=8.0Hz,8H),1.28(s,12H). 13 CNMR (100MHz, CD 3 OD)δ164.9,...
Embodiment 2
[0034] Solution preparation of fluorescent probe interacting with GSH
[0035] A certain amount of fluorescent probe was dissolved in water to obtain a concentration of 1.0×10 -4 mol L -1 Probe stock solution. Dissolve a certain amount of GSH in water, transfer it to a 500mL volumetric flask, add water to the mark, and obtain a concentration of 1.0×10 -2 mol L -1 GSH. will be 1.0×10 -2 mol L -1 The GSH solution was gradually diluted with water to obtain 1.0 x 10 -3 -1.0×10 -8 mol L -1 GSH aqueous solution. Add 1.0mL of the probe stock solution and 1.0mL of GSH aqueous solution into a 10mL volumetric flask, and dilute to the volume with buffer solution to obtain a concentration of 1.0×10 -5 mol L -1 fluorescent probe and 1.0 x 10 -3 -1.0×10 - 8 mol L -1 GSH mixed with the solution to be tested.
Embodiment 3
[0037] Measurement of Fluorescence Spectrum of Interaction of Fluorescent Probe with GSH
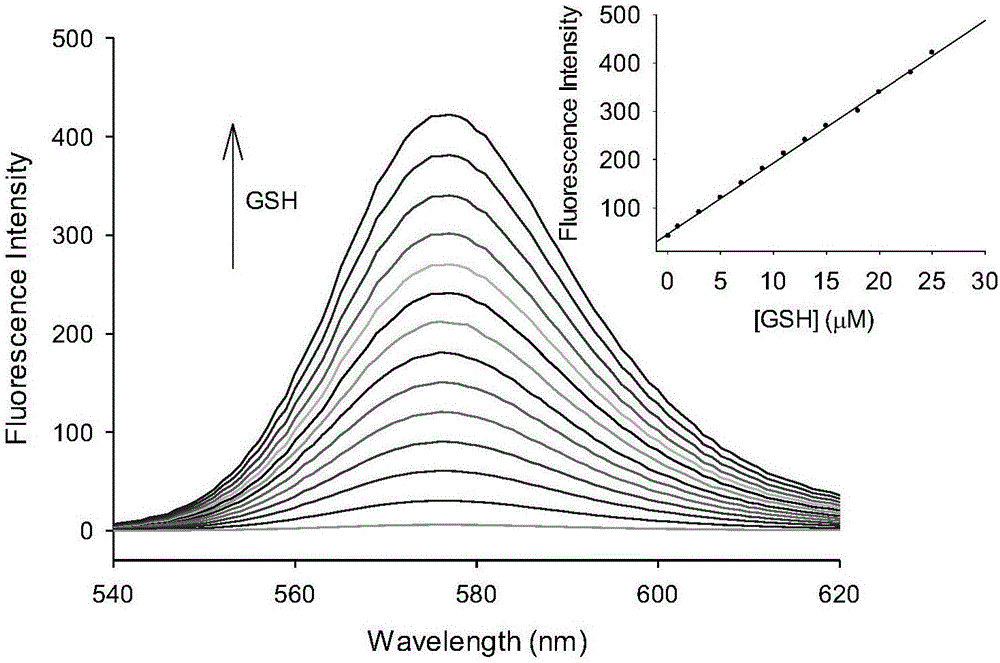
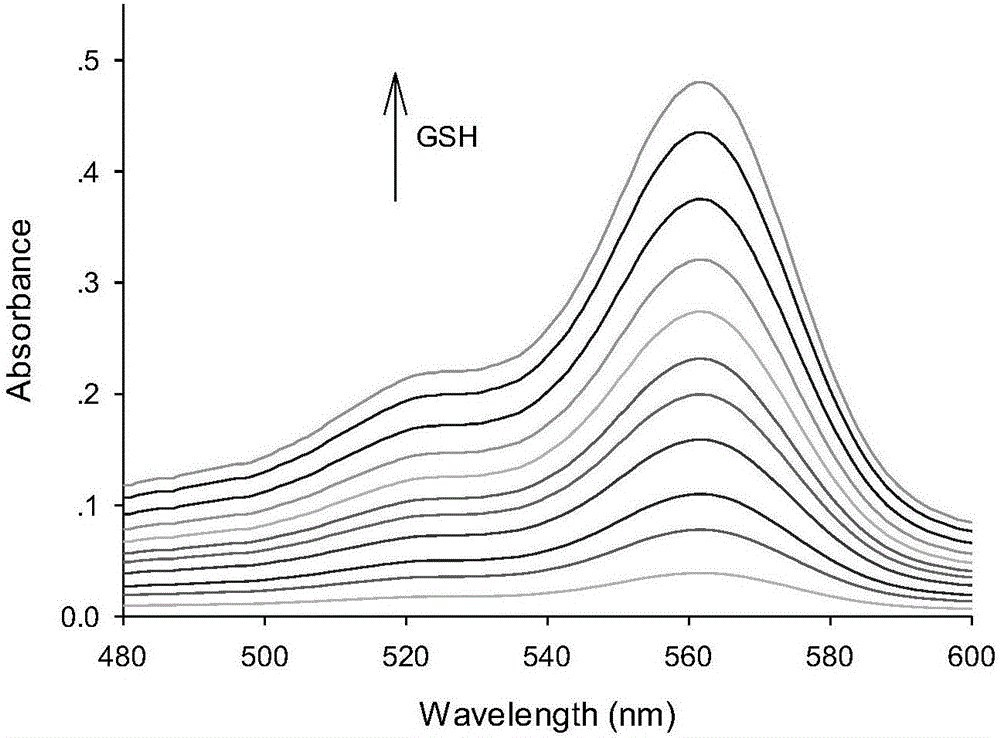
[0038] A buffer solution with a pH value of 7.4 was used as a solvent to measure the fluorescence spectrum of the fluorescent probe interacting with GSH, and the results were as follows: figure 1 . The concentration of the fluorescent probe is 10μM, the concentration of GSH is 0, 0.08, 1.0, 2.0, 3.0, 5.0, 7.0, 9.0, 11.0, 13.0, 15.0, 20.0, 23.0, 25.0μM, the excitation wavelength is fixed at 510nm, and the emission wavelength The range is 530-650nm, and the slit width is 5.0nm / 5.0nm. From figure 1 It can be seen that before adding GSH, the fluorescent probe has no fluorescence emission peak at 576 nm. With the addition of GSH, the emission peak at 576nm is greatly enhanced, and with the increase of GSH concentration, the fluorescence intensity of the probe is continuously enhanced. When 25 μM GSH is added, the fluorescence intensity is enhanced to 69 without GSH. times. This is becaus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com