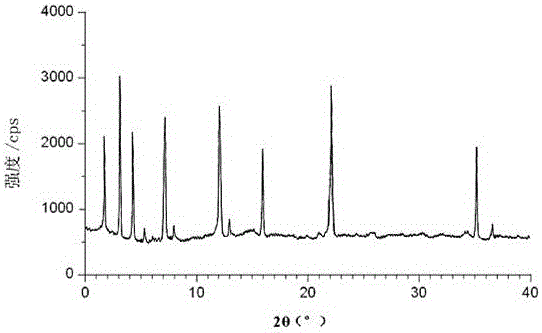
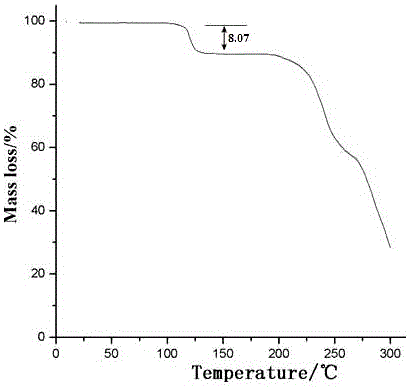
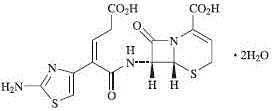
Method for preparing medicine ceftibuten crystal compound for treating surgical infection
A crystalline compound, the technology of cefbutene, applied in the field of medicine, can solve the problems of impurities, high polymer content, poor stability, etc., and achieve the effects of low polymer content, good stability and good clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 ceftibuten crystal compound
[0041] 1) Prepare mixed solution A by mixing dimethylformamide and water at a volume ratio of 4:1;
[0042] 2) Take the ceftibuten raw material, add the mixed solution A prepared in step 1), wherein the ratio of the volume of the mixed solution A to the mass of ceftibuten is 8ml:1g, stir to dissolve it all and pour it into the obtained solution Add activated carbon for decolorization and filtration to obtain a clear solution;
[0043] 3) Prepare mixed solution B with isopropyl ether and isopropanol at a volume ratio of 3.5:1;
[0044] 4) At room temperature, add mixed solution B to the clear solution obtained in step 2) under an ultrasonic field with a power of 0.5KW, wherein the volume of mixed solution B is 6 times the volume of mixed solution A, and turn off the ultrasonic field after adding. The temperature was lowered to 3° C. and allowed to stand for 2 hours to precipitate crystals, which were then dr...
Embodiment 2
[0048] The preparation of embodiment 2 ceftibuten crystal compound
[0049] 1) Prepare mixed solution A by mixing dimethylformamide and water at a volume ratio of 4:1;
[0050] 2) Take the ceftibuten raw material, add the mixed solution A prepared in step 1), wherein the ratio of the volume of the mixed solution A to the mass of ceftibuten is 9ml:1g, stir to dissolve it all, and pour it into the obtained solution Add activated carbon for decolorization and filtration to obtain a clear solution;
[0051] 3) Prepare mixed solution B with isopropyl ether and isopropanol at a volume ratio of 3.5:1;
[0052] 4) At room temperature, add mixed solution B to the clear solution obtained in step 2) under an ultrasonic field with a power of 0.6KW, wherein the volume of mixed solution B is 7 times the volume of mixed solution A, and turn off the ultrasonic field after adding. The temperature was lowered to 3° C. and allowed to stand for 2 hours to precipitate crystals, which were then d...
Embodiment 3
[0054] The preparation of embodiment 3 ceftibuten crystal compound
[0055] 1) Prepare mixed solution A by mixing dimethylformamide and water at a volume ratio of 4:1;
[0056] 2) Take the ceftibuten raw material, add the mixed solution A prepared in step 1), wherein the ratio of the volume of the mixed solution A to the mass of ceftibuten is 10ml:1g, stir to dissolve it all, and pour it into the obtained solution Add activated carbon for decolorization and filtration to obtain a clear solution;
[0057] 3) Prepare mixed solution B with isopropyl ether and isopropanol at a volume ratio of 3.5:1;
[0058] 4) At room temperature, add mixed solution B to the clear solution obtained in step 2) under an ultrasonic field with a power of 0.6KW, wherein the volume of mixed solution B is 8 times the volume of mixed solution A, and turn off the ultrasonic field after adding. The temperature was lowered to 3° C. and allowed to stand for 2 hours to precipitate crystals, which were then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com