N-acetyl glutamic acid kinase mutant and application thereof
A glutamic acid kinase and mutant technology, applied in the field of bioengineering, can solve the problems of weak catalytic activity, incomplete release, poor thermal stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Construction of Mutant Expression Plasmid and Obtaining of Recombinant Corynebacterium bacillus
[0025] According to argB published in Chinese patent CN201610286036.X F91H On the basis of the gene sequence, design primers at both ends of the gene encoding N-acetylglutamate kinase, and then design PCR point mutation intermediate primers according to the amino acid site to be mutated:
[0026] The mutant gene was amplified in vitro by overlap extension PCR.
[0027] The primers used for site-directed mutagenesis were:
[0028] PSD-argB F: 5'-CGCGAATTCAAAGGAGGGAAATCTTTTATGAATGACTTGAT
[0029] CAAAG(EcoR I)-3'
[0030] PargB R: 5'-CCAAGCTTTTACAGTTCCCCATCCTTG(Hind III)-3'
[0031] Parg B E19Y Rm: 5'-CATGGCAACGCNNNAGCGAGGACATTT-3'
[0032] Parg B I74V Fm: 5'-CGGTGGTGGACCTCAGGTTTCTGAGATGC-3'
[0033] Parg B I74V Rm: 5'-GCATCTCAGAAACCTGAGGTCCACCACCG-3'
[0034] Parg B K234T Fm: 5'-GTG TCCAAGATCA CTGCCACCGA GCTG-3'
[0035] Parg B K234T Rm: 5'-CAGCTC...
Embodiment 2
[0037] Example 2 Expression of mutant N-acetylglutamate kinase and purification of Ni-NTA
[0038] Inoculate the recombinants stored in cryopreserved tubes into LBG medium containing kanamycin (final concentration: 50 μg / mL), culture with shaking at 30°C overnight, transfer at 1% inoculum size, and culture at 30°C until the OD is about 0.6- 0.8, add human IPTG to a final concentration of 1mmol / L, and induce expression overnight. Centrifuge the overnight induced expression bacterial solution at 10000r / min, 4°C for 15min, collect the bacterial cells, suspend the bacterial cells with Tris-HCI (pH8.0) buffer, break the cells by ultrasonic, and then filter through a 0.45μm filter membrane, select the expression The vector pET-28a contains 6His-Tag, NAGK is purified by Ni-NTA, and the pure NAGK enzyme protein is analyzed by SDS-PAGE, and a specific band with a molecular weight of about 33kDa is detected. The pure NAGK enzyme is used for protein The concentration and enzyme activity...
Embodiment 3
[0039] Example 3 The Feedback Inhibition Effect of Final Product L-Arginine on CcNAGK Wild Type and Mutants
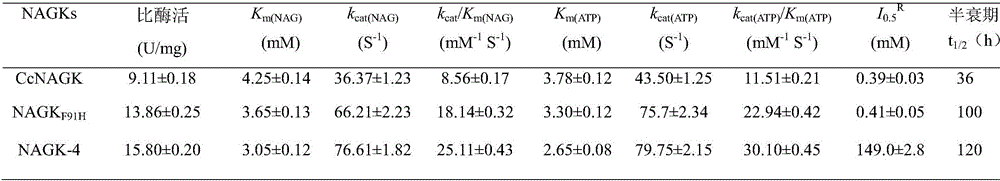
[0040] For the effect of the addition of L-arginine on the enzyme activity of CcNAGK, different concentrations (0-50 mM) of L-arginine were added to the enzymatic reaction system, and then the residual enzyme activity was measured. When the concentration of added L-arginine is 0, the enzyme activity measured is 100%. As the concentration of added L-arginine increases, the enzyme activity will decrease. When the enzyme activity drops to the initial enzyme activity Half of the time, we call the concentration of L-arginine added at this time as the half-inhibition constant, and the symbol is I 0.5 R (R stands for L-arginine). It was found that: I of the mutant E19Y 0.5 R The value is increased by about 380 times. Analysis of the three-dimensional structure of CcNAGK found that the E19 site is located at the entrance of the L-arginine binding pocket (Arginine-ring inc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com