Vanillin derivative containing dithioacetals, preparation method and purpose thereof
A technology of dithioacetal and vanillin, which is applied in the chemical field, can solve the problem of anti-plant virus that has not been reported, achieve good therapeutic and protective effects, and improve the effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
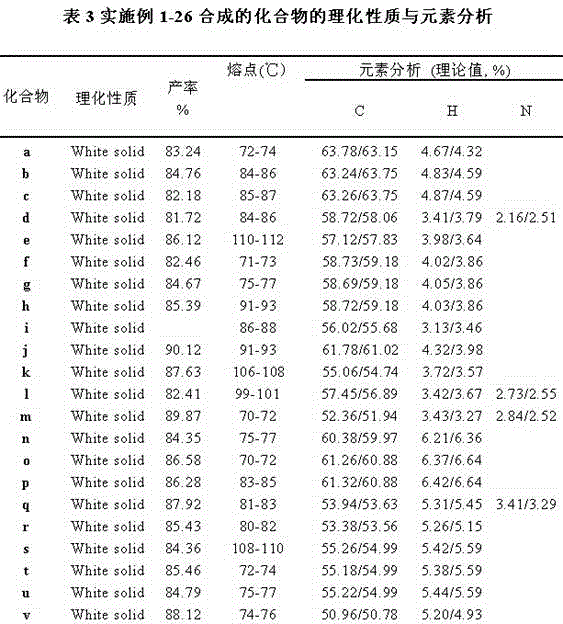
Examples
Embodiment 1
[0027] ((((4-phenyl) oxygen)-3-methoxyphenyl) methylene) two (4-chlorophenyl) dithioacetal synthetic (compound number is a), comprising the following steps:
[0028] (1) Synthesis of 4-(benzyloxy)-3-methoxybenzaldehyde:
[0029] Add vanillin (1.52 g, 10 mmol) and benzyl chloride (1.26 g, 10 mmol) into a 100 mL three-neck flask and add 40 mL of acetonitrile to dissolve the solid, add anhydrous K 2 CO 3 (2.76 g, 20 mmol), the reaction system is light yellow turbidity (K 2 CO 3 undissolved), refluxed and stirred, followed the reaction process by TLC (the developing solvent was petroleum ether:ethyl acetate=3:1, V / V), after the raw material point disappeared, the reaction was stopped, the solvent was spin-dried, and 50 mL H 2 O was stirred at room temperature to separate out a white solid, and recrystallized from ethanol to obtain a white solid;
[0030] (2) ((((4-phenyl) oxygen)-3-methoxyphenyl) methylene) two (4-chlorophenyl) dithioacetal synthesis:
[0031] Add 4-(benzylox...
Embodiment 2
[0033] ((4-((3-methylphenyl) oxygen)-3-methoxyphenyl) methylene) two (4-chlorophenyl) dithioacetal synthesis (compound number is b), including The following steps:
[0034] (1) Synthesis of 4-((3-methylphenyl) oxygen)-3-methoxybenzaldehyde:
[0035] Synthesize as embodiment 1 (1) method and condition, difference is that 3-methyl benzyl chloride is raw material;
[0036] (2) ((4-((3-methylphenyl) oxygen)-3-methoxyphenyl) methylene) two (4-chlorophenyl) dithioacetal synthesis:
[0037] Synthesize as in Example 1(2) method and conditions, the difference is that 4-((3-methylphenyl)oxy)-3-methoxybenzaldehyde is the raw material.
Embodiment 3
[0039] Synthesis of ((4-((4-methylphenyl)oxy)-3-methoxyphenyl)methylene)bis(4-chlorophenyl)dithioacetal (compound number c), including The following steps:
[0040] (1) Synthesis of 4-((4-methylphenyl) oxygen)-3-methoxybenzaldehyde:
[0041] Synthesize as embodiment 1 (1) method and condition, difference is that 4-methyl benzyl chloride is raw material;
[0042] (2) ((4-((4-methylphenyl) oxygen)-3-methoxyphenyl) methylene) two (4-chlorophenyl) dithioacetal synthesis:
[0043] Synthesize as in Example 1 (2) method and conditions, the difference is that 4-((4-methylphenyl)oxy)-3-methoxybenzaldehyde is the raw material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com