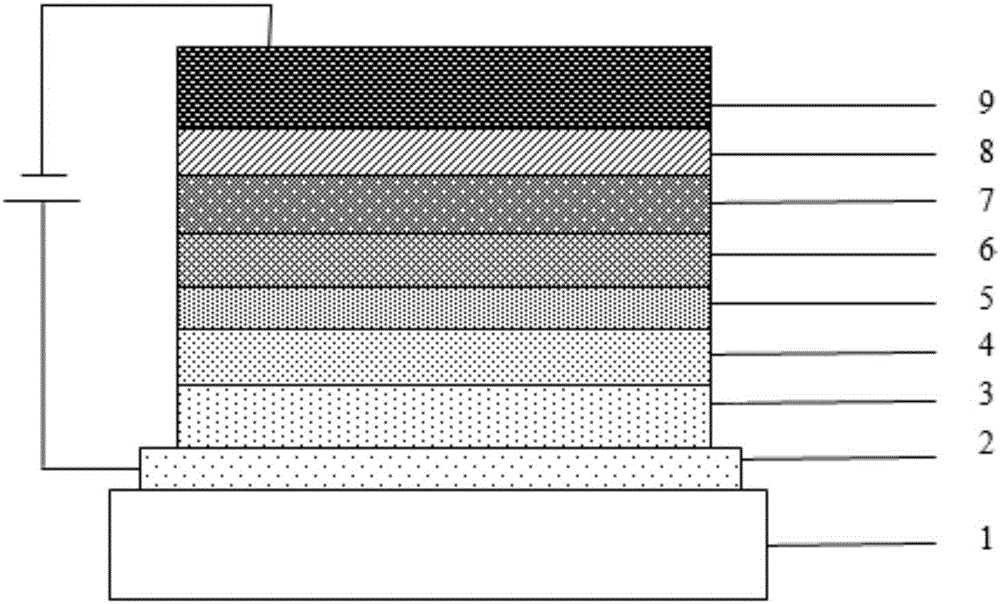
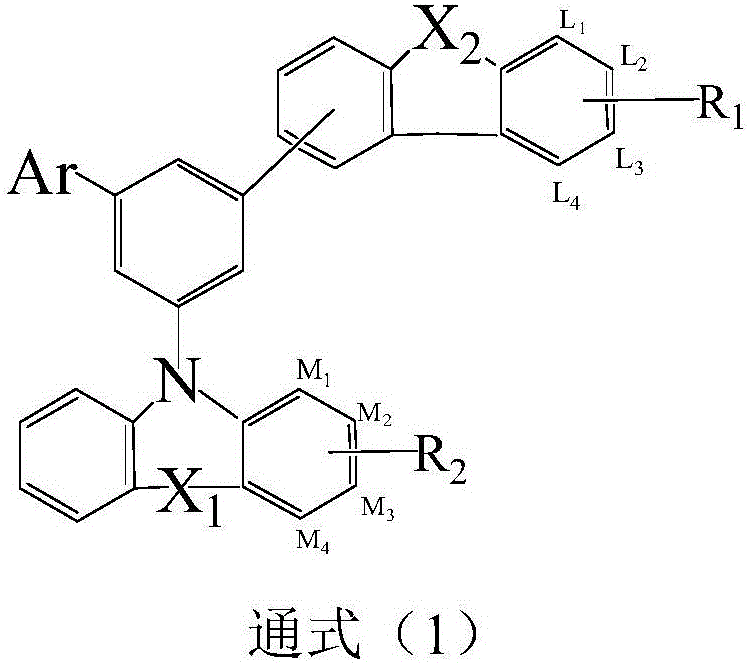
Photoelectric material with average benzene as core and application thereof
A photoelectric material, phenyl technology, applied in luminescent materials, circuits, electrical components, etc., can solve different problems, achieve the effect of improving triplet state energy level, improving stability, and good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the synthesis of compound 3
[0050] (1) Synthesis of intermediates
[0051]
[0052] 1-Phenyl-3-chloro-5-bromobenzene (3.5g, 10mmol), dibenzofuran-4-boronic acid (2.3g, 11mmol), sodium carbonate (5.1g, 48mmol), Pd 2 (dba) 3 (0.4g, 0.4mmol), toluene, ethanol, each 50ml of water were added in the reaction flask successively, refluxed reaction under nitrogen protection for 10 hours, cooled to room temperature, liquid separation, the aqueous layer was extracted with ethyl acetate, combined organic layer, respectively Wash with saturated brine and water, dry the organic layer with magnesium sulfate, filter, spin the filtrate, and pass through a silica gel column to obtain 3 g of the product with a HPLC purity of 99.2%.
[0053] (2) Synthesis of compound 3
[0054]
[0055] Raw material S1 (3.5g, 10.0mmol), raw material S2 (3.1g, 10.2mmol) were added to the reaction flask, sodium tert-butoxide (1.2g, 12mmol), Pd 2 (dba) 3 (0.1g, 0.1mmol), 50ml of tol...
Embodiment 2
[0057] Embodiment 2: the synthesis of compound 9
[0058] (1) Synthesis of intermediates
[0059]
[0060] 1-Phenyl-3-chloro-5-bromobenzene (3.5g, 10mmol), dibenzofuran-4-boronic acid (2.3g, 11mmol), sodium carbonate (5.1g, 48mmol), Pd 2 (dba) 3 (0.4g, 0.4mmol), toluene, ethanol, each 50ml of water were added in the reaction flask successively, refluxed reaction under nitrogen protection for 10 hours, cooled to room temperature, liquid separation, the aqueous layer was extracted with ethyl acetate, combined organic layer, respectively Wash with saturated brine and water, dry the organic layer with magnesium sulfate, filter, spin the filtrate, pass through a silica gel column to obtain 3g of product, HPLC purity 99.2%
[0061] (2) Synthesis of compound 9
[0062]
[0063] Raw material S1 (3.5g, 10.0mmol), raw material S3 (3.1g, 10.2mmol) were added to the reaction flask, sodium tert-butoxide (1.2g, 12mmol), Pd 2 (dba) 3 (0.1g, 0.1mmol), 50ml of toluene were sequentia...
Embodiment 3
[0065] Embodiment 3: the synthesis of compound 12
[0066] (1) Synthesis of intermediates
[0067]
[0068] 1-Phenyl-3-chloro-5-bromobenzene (3.5g, 10mmol), dibenzofuran-4-boronic acid (2.3g, 11mmol), sodium carbonate (5.1g, 48mmol), Pd 2 (dba) 3 (0.4g, 0.4mmol), toluene, ethanol, each 50ml of water were added in the reaction flask successively, refluxed reaction under nitrogen protection for 10 hours, cooled to room temperature, liquid separation, the aqueous layer was extracted with ethyl acetate, combined organic layer, respectively Wash with saturated brine and water, dry the organic layer with magnesium sulfate, filter, spin the filtrate, pass through a silica gel column to obtain 3g of product, HPLC purity 99.2%
[0069] (2) Synthesis of Compound 12
[0070]
[0071] Raw material S1 (3.5g, 10.0mmol), raw material S4 (3.1g, 10.2mmol) was added to the reaction flask, sodium tert-butoxide (1.2g, 12mmol), Pd 2 (dba) 3 (0.1g, 0.1mmol), 50ml of toluene were sequenti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com