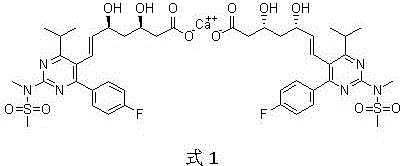
Method for preparing heptenoic acid cyclopentyl ester derivative
A technology of cyclopentyl heptenoate and its derivatives, which is applied in the field of preparation of cyclopentyl heptenoate derivatives, can solve the problems of unfavorable high-quality products and poor effects, and achieve high overall yield of the route and increase Safety, effect of short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of Intermediate I:
[0033] Add 500mL methanol and 30g (4R-cis)-6-[(acetoxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester into the reaction flask and 27.8g of potassium carbonate, stirred and dissolved, reacted at 20-25°C, and monitored the reaction by TLC. After the reaction is over, add 10mL of glacial acetic acid to the reaction solution, stir, heat up to 45-50°C and distill methanol off under reduced pressure, then add 300mL of toluene and 200mL of water, stir, and let stand to separate the liquids; the water layer is then extracted with 200mL of toluene, The organic phases were combined, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated to dryness under reduced pressure at 45-50°C to obtain 28.2 g of the product, which was directly subjected to the next reaction.
[0034] Synthesis of Intermediate II:
[0035] Add 28.2g of intermediate I and 300mL of methanol into the reaction flask, stir to ...
Embodiment 2
[0043] Synthesis of Intermediate I:
[0044] Add 700mL isopropanol, 45g (4R-cis)-6-[(acetoxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert- Butyl ester and 12.5g of sodium hydroxide were stirred and dissolved, reacted at 20-25°C, and monitored by TLC. After the reaction is over, add 20mL of glacial acetic acid to the reaction solution, stir, heat up to 45-50°C and distill off isopropanol under reduced pressure, then add 500mL of ethyl acetate and 300mL of water, stir, and let stand to separate the liquid; Extract with 500mL of ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to dryness under reduced pressure at 45-50°C to obtain 42.3g of the product, which is directly subjected to the next reaction.
[0045] Synthesis of Intermediate II:
[0046] Add 42.3g of intermediate I and 300mL of isopropanol into the reaction flask, stir to dissolve, add 10.3g of lithium hydroxide, heat up to 45-50°C; k...
Embodiment 3
[0054] Synthesis of Intermediate I:
[0055] Add 700mL ethanol, 38g (4R-cis)-6-[(acetoxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester in the reaction flask and 6.4g of lithium hydroxide, stirred and dissolved, reacted at 20-25°C, and monitored the reaction by TLC. After the reaction is completed, add 15 mL of glacial acetic acid to the reaction solution, stir, heat up to 45-50 °C and distill off ethanol under reduced pressure, then add 450 mL of toluene and 250 mL of water, stir, and let stand to separate the liquids; the water layer is then extracted with 400 mL of toluene, The organic phases were combined, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated to dryness under reduced pressure at 45-50°C to obtain 35.9 g of the product, which was directly subjected to the next reaction.
[0056] Synthesis of Intermediate II:
[0057] Add 35.9g of intermediate I and 300mL of acetonitrile into the reaction flask, stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com