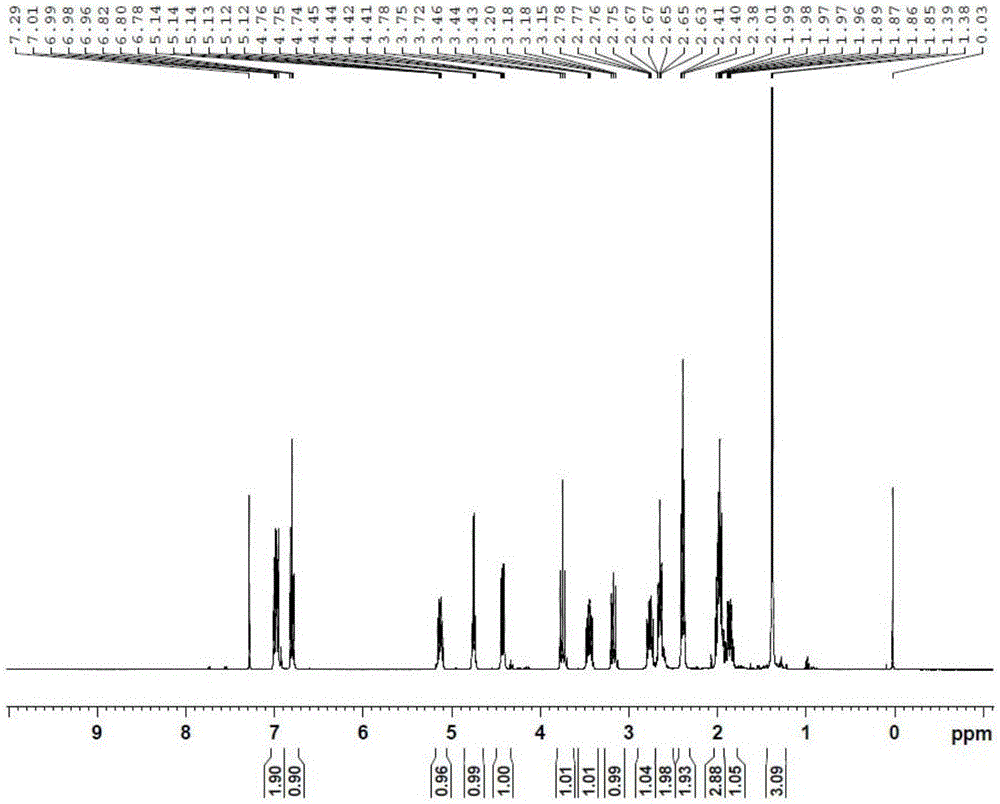
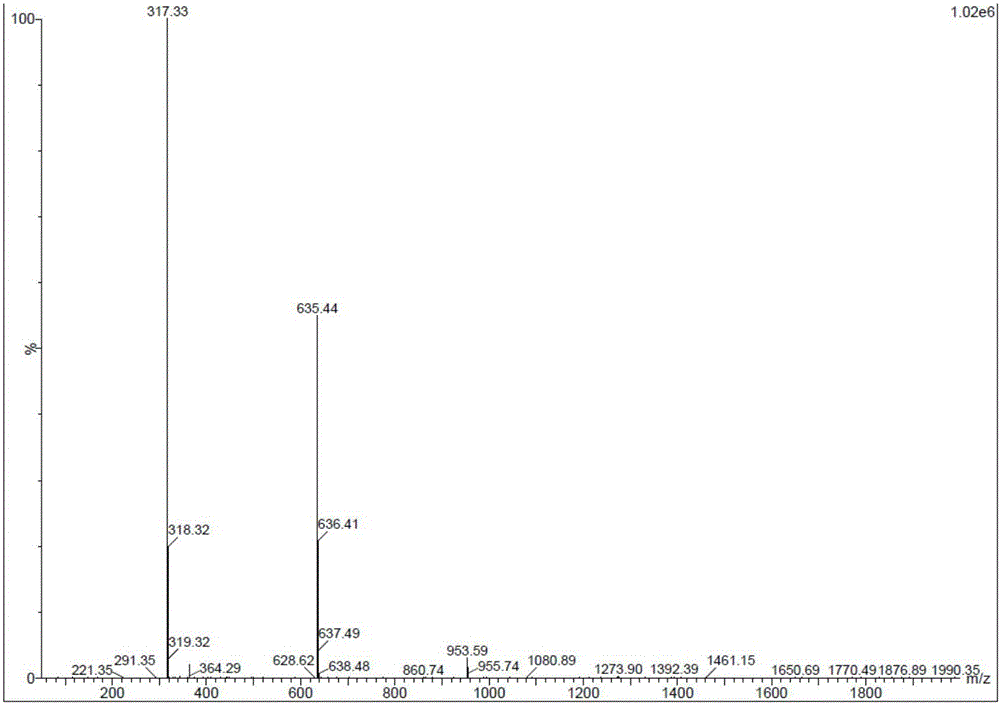
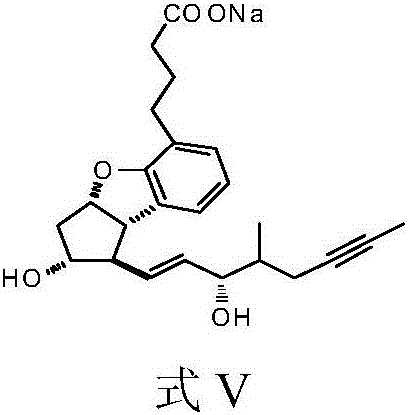
Preparation method of Beraprost and its salt
A technology of beraprost and formula, applied in organic chemistry methods, bulk chemical production, organic chemistry, etc., can solve problems not related to the preparation method of beraprost sodium, achieve optimized preparation process route, facilitate scale-up, and stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1I-1
[0071] Embodiment 1.I-1 (R 1 =Bn, X=Cl) preparation
[0072] Add 14 g (40.5 mmol) of II (X=Cl) to a 500 ml three-necked flask, add 140 ml of dry tetrahydrofuran, and cool to -78°C. Under nitrogen protection, 19.5 ml of n-butyl lithium in tetrahydrofuran solution (48.6 mmol) was slowly added dropwise to the bottle. After the addition was complete, stirring was continued at -78°C for 20 minutes. Start to add 10.8g of 4-benzyloxy hydroxybutyraldehyde tetrahydrofuran solution (60.75mmol) dropwise, after the dropwise addition, continue to stir at -78°C for 20 minutes, then rise to -40°C and add 30ml of saturated ammonium chloride solution dropwise, stir, and rise to At room temperature, add water, extract 3 times with ethyl acetate, collect the organic phase, wash the organic phase once with saturated brine, dry the organic phase with anhydrous sodium sulfate, filter the organic phase and concentrate under reduced pressure to obtain the crude product I-1, column chromatography Af...
Embodiment 2
[0073] Embodiment 2.I-1 (R 1 =TBS, X=Cl) preparation
[0074] Add 5.4 g (15.6 mmol) of II (X=Cl) into a 250 ml three-necked flask, add 54 ml of dry tetrahydrofuran, and cool to -78°C. Under nitrogen protection, 7.5 ml of a tetrahydrofuran solution (18.7 mmol) of n-butyllithium was slowly added dropwise to the bottle, and after the addition was complete, stirring was continued at -78°C for 20 minutes. Start to add dropwise 4.7g of 4-tert-butyldimethylsiloxy hydroxybutyraldehyde in tetrahydrofuran (23.4mmol). After the dropwise addition, continue to stir at -78°C for 20 minutes, then add dropwise 10ml of saturated ammonium chloride solution to -40°C , stirred, raised to room temperature, added water, extracted 3 times with ethyl acetate, collected the organic phase, washed the organic phase with saturated brine once, dried the organic phase with anhydrous sodium sulfate, filtered the organic phase and concentrated under reduced pressure to obtain I-1 The crude product was puri...
Embodiment 3
[0075] Embodiment 3.I-1 (R 1 =TBS, X=Br) preparation
[0076] Add 3.2 g (8.2 mmol) of II (X=Br) into a 100 ml three-necked flask, add 32 ml of dry tetrahydrofuran, and cool to -78°C. Under nitrogen protection, 3.9 ml of a tetrahydrofuran solution (9.8 mmol) of n-butyllithium was slowly added dropwise to the bottle. After the addition was complete, stirring was continued at -78°C for 20 minutes. Start to add dropwise 2.5g of 4-tert-butyldimethylsiloxy hydroxybutyraldehyde in tetrahydrofuran (12.3mmol). After the dropwise addition, continue to stir at -78°C for 20 minutes, then rise to -40°C and add 10ml of saturated ammonium chloride solution dropwise , stirred, raised to room temperature, added water, extracted 3 times with ethyl acetate, collected the organic phase, washed the organic phase with saturated brine once, dried the organic phase with anhydrous sodium sulfate, filtered the organic phase, and concentrated under reduced pressure to obtain I- 1 The crude product was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com